Performance of Three SARS-CoV-2 Immunoassays, Three Rapid Lateral Flow Tests, and a Novel Bead-Based Affinity Surrogate Test for the Detection of SARS-CoV-2 Antibodies in Human Serum
- PMID: 33962959
- PMCID: PMC8373215
- DOI: 10.1128/JCM.00319-21
Performance of Three SARS-CoV-2 Immunoassays, Three Rapid Lateral Flow Tests, and a Novel Bead-Based Affinity Surrogate Test for the Detection of SARS-CoV-2 Antibodies in Human Serum
Abstract
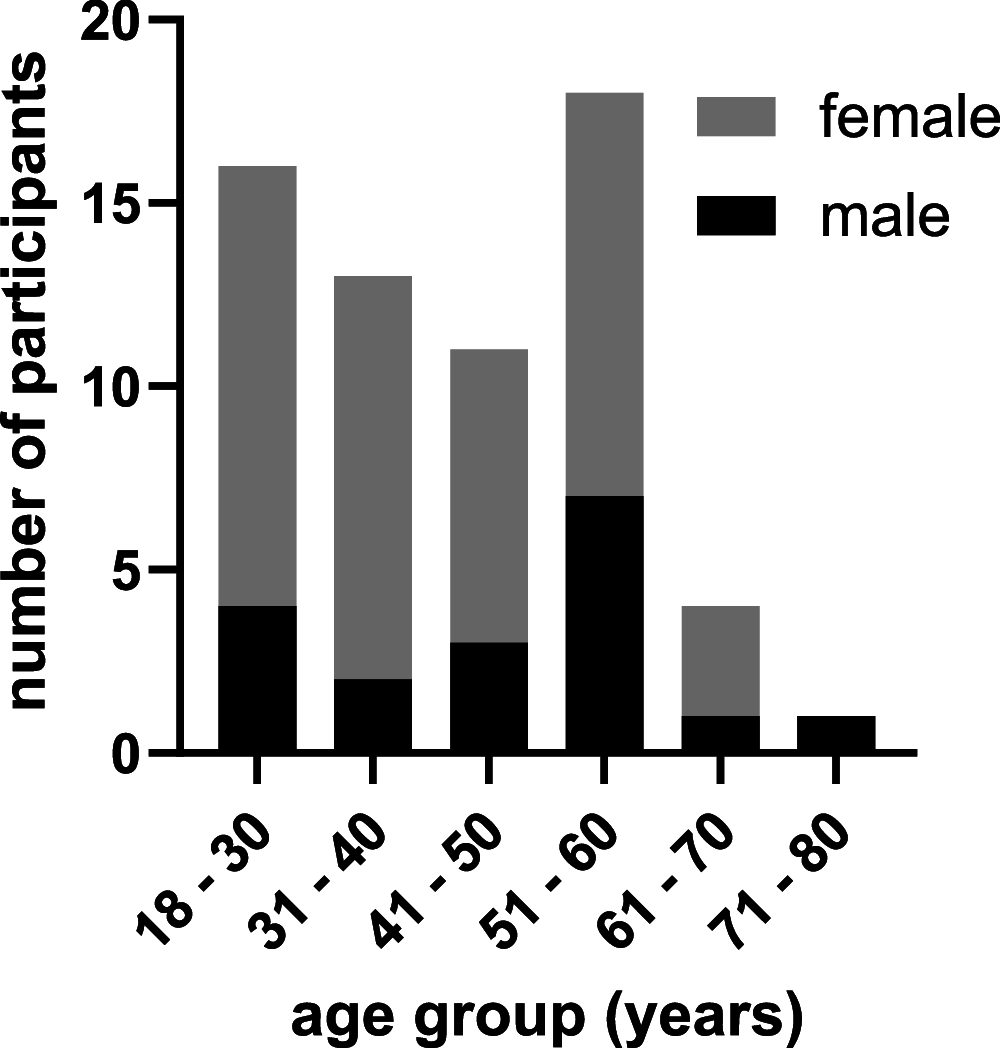
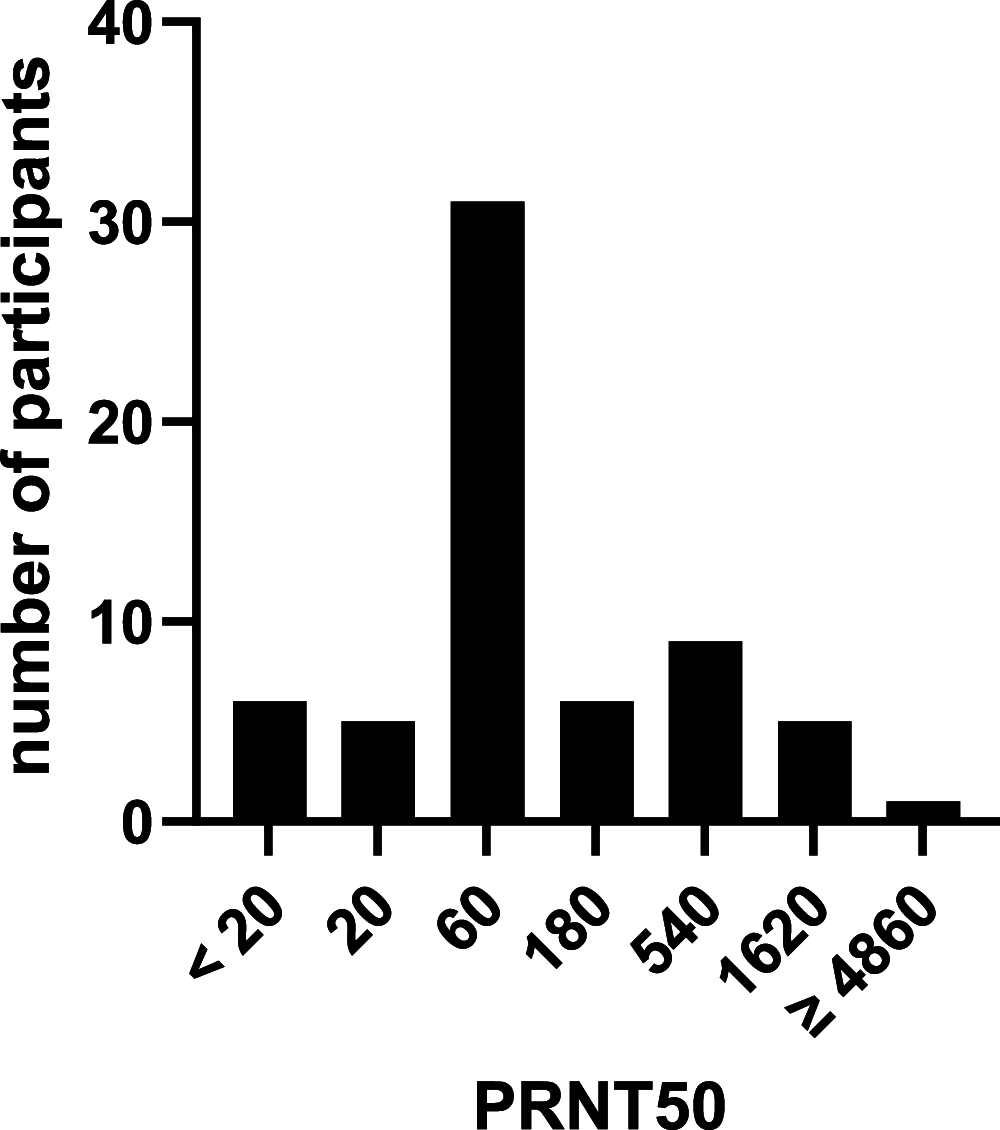
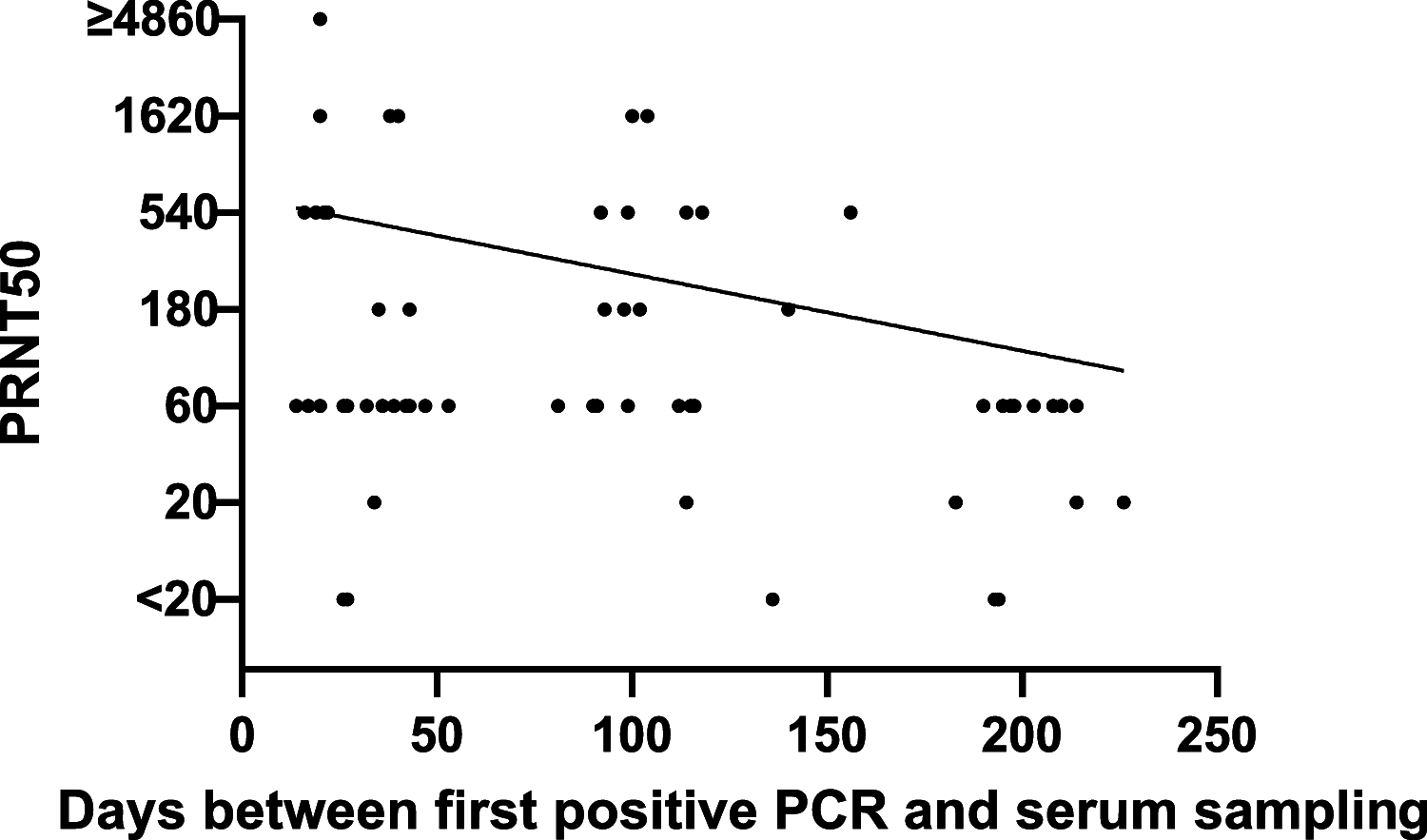
For the control of immunity in COVID-19 survivors and vaccinated subjects, there is an urgent need for reliable and rapid serological assays. Based on samples from 63 COVID-19 survivors up to 7 months after symptom onset, and on 50 serum samples taken before the beginning of the pandemic, we compared the performances of three commercial immunoassays for the detection of SARS-CoV-2 IgA and IgG antibodies (Euroimmun SARS-COV-2 IgA/IgG, Mikrogen recomWell SARS-CoV-2 IgA/IgG, and Serion ELISA agile SARS-CoV-2 IgA/IgG) and three rapid lateral flow (immunochromatographic) tests (Abbott PanBio COVID-19 IgG/IgM, Nadal COVID-19 IgG/IgM, and Cleartest Corona 2019-nCOV IgG/IgM) with a 50% plaque-reduction neutralization test (PRNT50) representing the gold standard. Fifty-seven out of 63 PCR-confirmed COVID-19 patients (90%) showed neutralizing antibodies. The sensitivity of the seven assays ranged from 7.0% to 98.3%, and the specificity ranged from 86.0% to 100.0%. Only one commercial immunoassay showed a sensitivity and specificity of greater than 98%.
Keywords: COVID-19; ELISA; SARS-CoV-2; flow cytometry bead-based surrogate test; lateral flow assay; plaque-reduction neutralization test.
Figures
Similar articles
-
Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech).J Clin Virol. 2020 Aug;129:104511. doi: 10.1016/j.jcv.2020.104511. Epub 2020 Jun 15. J Clin Virol. 2020. PMID: 32593133 Free PMC article.
-
Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation.J Clin Virol. 2020 Aug;129:104512. doi: 10.1016/j.jcv.2020.104512. Epub 2020 Jun 15. J Clin Virol. 2020. PMID: 32563180 Free PMC article.
-
Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies.J Clin Virol. 2020 Jul;128:104413. doi: 10.1016/j.jcv.2020.104413. Epub 2020 May 5. J Clin Virol. 2020. PMID: 32403010 Free PMC article.
-
Seropositivity rate and diagnostic accuracy of serological tests in 2019-nCoV cases: a pooled analysis of individual studies.Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10208-10218. doi: 10.26355/eurrev_202010_23243. Eur Rev Med Pharmacol Sci. 2020. PMID: 33090430 Review.
-
Increasing the Efficiency of a National Laboratory Response to COVID-19: a Nationwide Multicenter Evaluation of 47 Commercial SARS-CoV-2 Immunoassays by 41 Laboratories.J Clin Microbiol. 2021 Aug 18;59(9):e0076721. doi: 10.1128/JCM.00767-21. Epub 2021 Aug 18. J Clin Microbiol. 2021. PMID: 34191578 Free PMC article. Review.
Cited by
-
A Comparative Study of Nine SARS-CoV-2 IgG Lateral Flow Assays Using Both Post-Infection and Post-Vaccination Samples.J Clin Med. 2022 Apr 8;11(8):2100. doi: 10.3390/jcm11082100. J Clin Med. 2022. PMID: 35456192 Free PMC article.
-
Longitudinal change in SARS-CoV-2 seroprevalence in 3-to 16-year-old children: The Augsburg Plus study.PLoS One. 2022 Aug 11;17(8):e0272874. doi: 10.1371/journal.pone.0272874. eCollection 2022. PLoS One. 2022. PMID: 35951611 Free PMC article.
-
Influencing factors of anti-SARS-CoV-2-spike-IgG antibody titers in healthcare workers: A cross-section study.J Med Virol. 2023 Jan;95(1):e28300. doi: 10.1002/jmv.28300. Epub 2022 Nov 18. J Med Virol. 2023. PMID: 36369641 Free PMC article.
-
Comparison of the Anti-SARS-CoV-2 Surrogate Neutralization Assays by TECOmedical and DiaPROPH-Med with Samples from Vaccinated and Infected Individuals.Viruses. 2022 Feb 3;14(2):315. doi: 10.3390/v14020315. Viruses. 2022. PMID: 35215912 Free PMC article.
-
A community based seroprevalence of SARS-CoV-2 antibodies in Somali Region, Eastern Ethiopia.Immun Inflamm Dis. 2024 Jan;12(1):e1148. doi: 10.1002/iid3.1148. Immun Inflamm Dis. 2024. PMID: 38270297 Free PMC article.
References
-
- World Health Organization. 2021. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland. https://covid19.who.int/. Accessed 7 February 2021.
-
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523. doi:10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2021. Interim guidelines for COVID-19 antibody testing. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g.... Accessed 17 March 2021.
-
- European Centre for Disease Prevention and Control. 11June2020. Diagnostic testing and screening for SARS-CoV-2. European Centre for Disease Prevention and Control, Solna, Sweden. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

