Performance of Three SARS-CoV-2 Immunoassays, Three Rapid Lateral Flow Tests, and a Novel Bead-Based Affinity Surrogate Test for the Detection of SARS-CoV-2 Antibodies in Human Serum
- PMID: 33962959
- PMCID: PMC8373215
- DOI: 10.1128/JCM.00319-21
Performance of Three SARS-CoV-2 Immunoassays, Three Rapid Lateral Flow Tests, and a Novel Bead-Based Affinity Surrogate Test for the Detection of SARS-CoV-2 Antibodies in Human Serum
Abstract
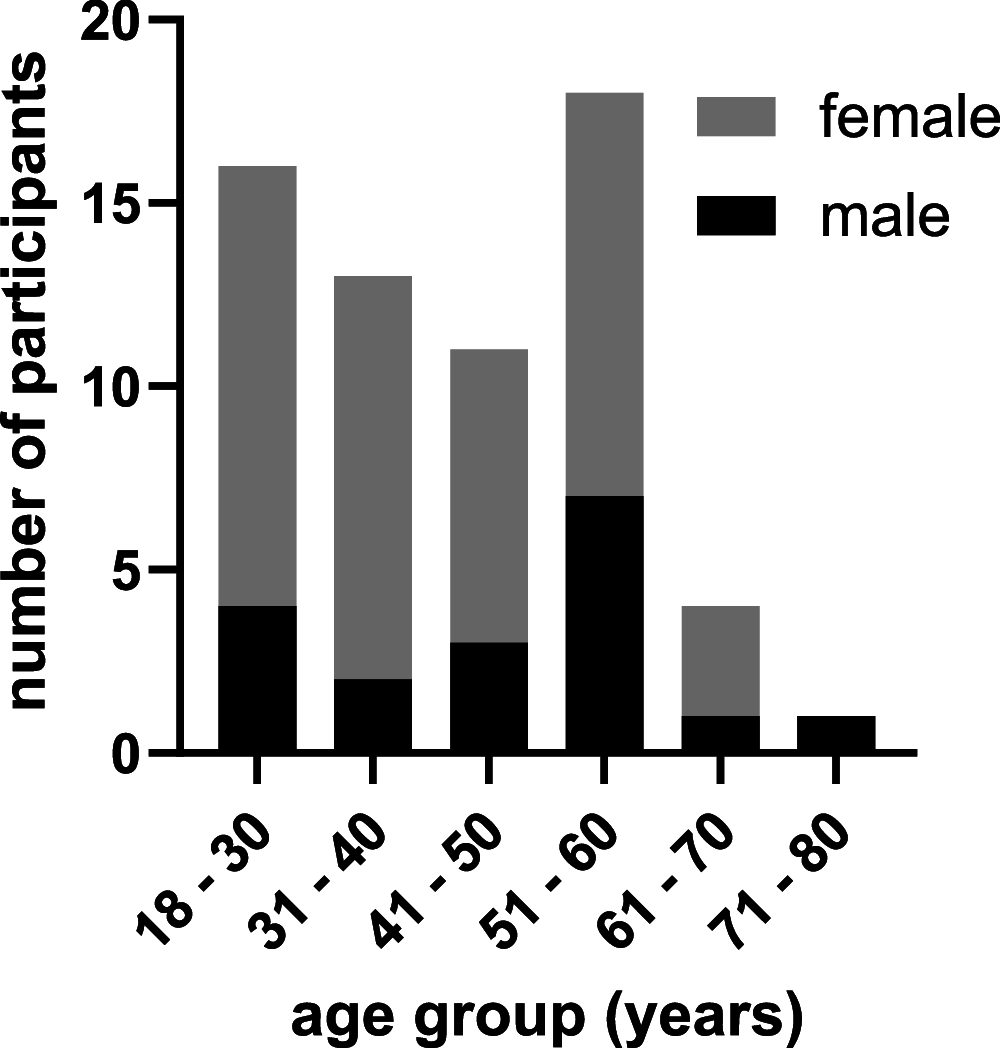
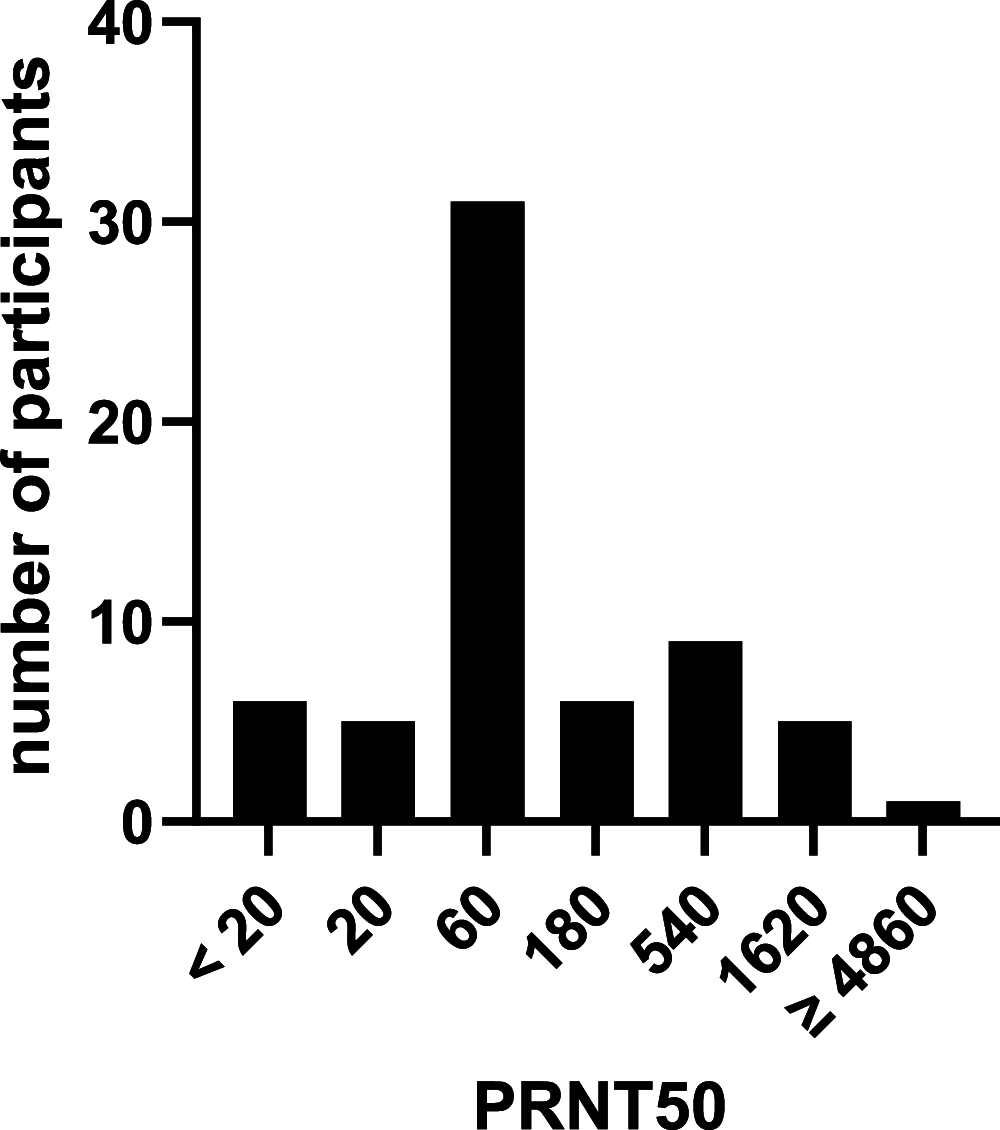
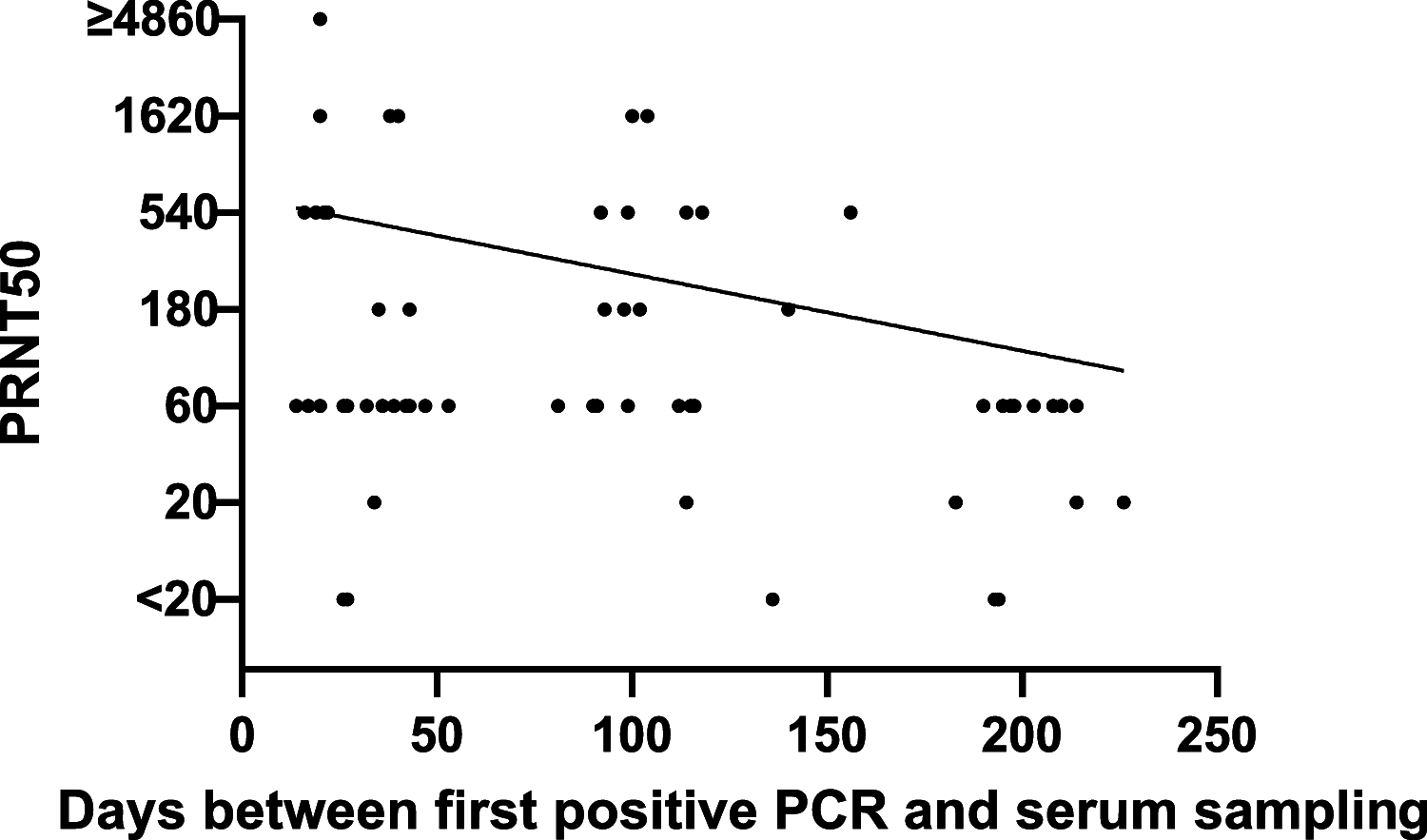
For the control of immunity in COVID-19 survivors and vaccinated subjects, there is an urgent need for reliable and rapid serological assays. Based on samples from 63 COVID-19 survivors up to 7 months after symptom onset, and on 50 serum samples taken before the beginning of the pandemic, we compared the performances of three commercial immunoassays for the detection of SARS-CoV-2 IgA and IgG antibodies (Euroimmun SARS-COV-2 IgA/IgG, Mikrogen recomWell SARS-CoV-2 IgA/IgG, and Serion ELISA agile SARS-CoV-2 IgA/IgG) and three rapid lateral flow (immunochromatographic) tests (Abbott PanBio COVID-19 IgG/IgM, Nadal COVID-19 IgG/IgM, and Cleartest Corona 2019-nCOV IgG/IgM) with a 50% plaque-reduction neutralization test (PRNT50) representing the gold standard. Fifty-seven out of 63 PCR-confirmed COVID-19 patients (90%) showed neutralizing antibodies. The sensitivity of the seven assays ranged from 7.0% to 98.3%, and the specificity ranged from 86.0% to 100.0%. Only one commercial immunoassay showed a sensitivity and specificity of greater than 98%.
Keywords: COVID-19; ELISA; SARS-CoV-2; flow cytometry bead-based surrogate test; lateral flow assay; plaque-reduction neutralization test.
Figures
References
-
- World Health Organization. 2021. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland. https://covid19.who.int/. Accessed 7 February 2021.
-
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2021. Interim guidelines for COVID-19 antibody testing. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g.... Accessed 17 March 2021.
-
- European Centre for Disease Prevention and Control. 11June2020. Diagnostic testing and screening for SARS-CoV-2. European Centre for Disease Prevention and Control, Solna, Sweden. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

