Half-life modeling of basic fibroblast growth factor released from growth factor-eluting polyelectrolyte multilayers
- PMID: 33963247
- PMCID: PMC8105364
- DOI: 10.1038/s41598-021-89229-w
Half-life modeling of basic fibroblast growth factor released from growth factor-eluting polyelectrolyte multilayers
Abstract
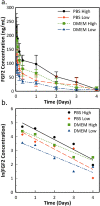
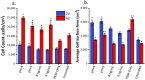
Growth factor-eluting polymer systems have been widely reported to improve cell and tissue outcomes; however, measurements of actual growth factor concentration in cell culture conditions are limited. The problem is compounded by a lack of knowledge of growth factor half-lives, which impedes efforts to determine real-time growth factor concentrations. In this work, the half-life of basic fibroblast growth factor (FGF2) was determined using enzyme linked immunosorbent assay (ELISA). FGF2 release from polyelectrolyte multilayers (PEMs) was measured and the data was fit to a simple degradation model, allowing for the determination of FGF2 concentrations between 2 and 4 days of culture time. After the first hour, the FGF2 concentration for PEMs assembled at pH = 4 ranged from 2.67 ng/mL to 5.76 ng/mL, while for PEMs assembled at pH = 5, the concentration ranged from 0.62 ng/mL to 2.12 ng/mL. CRL-2352 fibroblasts were cultured on PEMs assembled at pH = 4 and pH = 5. After 2 days, the FGF2-eluting PEM conditions showed improved cell count and spreading. After 4 days, only the pH = 4 assembly condition had higher cells counts, while the PEM assembled at pH = 5 and PEM with no FGF2 showed increased spreading. Overall, the half-life model and cell culture study provide optimal concentration ranges for fibroblast proliferation and a framework for understanding how temporal FGF2 concentration may affect other cell types.
Conflict of interest statement
The authors are inventors of a patent (US Patent 10731145) for coated cell culture apparatuses using polyelectrolyte multilayers that may be capable of controlled release.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

