Thyroid Hormone Deiodinases: Dynamic Switches in Developmental Transitions
- PMID: 33963379
- PMCID: PMC8248586
- DOI: 10.1210/endocr/bqab091
Thyroid Hormone Deiodinases: Dynamic Switches in Developmental Transitions
Abstract
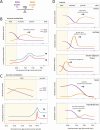
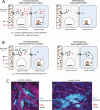
Thyroid hormones exert pleiotropic, essential actions in mammalian, including human, development. These actions depend on provision of thyroid hormones in the circulation but also to a remarkable extent on deiodinase enzymes in target tissues that amplify or deplete the local concentration of the primary active form of the hormone T3 (3,5,3'-triiodothyronine), the high affinity ligand for thyroid hormone receptors. Genetic analyses in mice have revealed key roles for activating (DIO2) and inactivating (DIO3) deiodinases in cell differentiation fates and tissue maturation, ultimately promoting neonatal viability, growth, fertility, brain development, and behavior, as well as metabolic, endocrine, and sensory functions. An emerging paradigm is how the opposing activities of DIO2 and DIO3 are coordinated, providing a dynamic switch that controls the developmental timing of a tissue response, often during neonatal and maturational transitions. A second paradigm is how cell to cell communication within a tissue determines the response to T3. Deiodinases in specific cell types, often strategically located near to blood vessels that convey thyroid hormones into the tissue, can regulate neighboring cell types, suggesting a paracrine-like layer of control of T3 action. We discuss deiodinases as switches for developmental transitions and their potential to influence tissue dysfunction in human thyroid disorders.
Keywords: central nervous system; deiodinase; development; neuroendocrine function; sensory system; thyroid hormone.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Osler W. Sporadic Cretinism in America. Transactions; Congress of American Physicians and Surgeons. 1897;4:169-206.
-
- Polak M, Legac I, Vuillard E, Guibourdenche J, Castanet M, Luton D. Congenital hyperthyroidism: the fetus as a patient. Horm Res. 2006;65(5):235-242. - PubMed
-
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

