Overexpression of thioredoxin m in chloroplasts alters carbon and nitrogen partitioning in tobacco
- PMID: 33963398
- PMCID: PMC8219043
- DOI: 10.1093/jxb/erab193
Overexpression of thioredoxin m in chloroplasts alters carbon and nitrogen partitioning in tobacco
Abstract
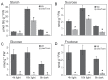
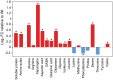
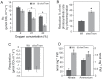
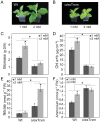
In plants, there is a complex interaction between carbon (C) and nitrogen (N) metabolism, and its coordination is fundamental for plant growth and development. Here, we studied the influence of thioredoxin (Trx) m on C and N partitioning using tobacco plants overexpressing Trx m from the chloroplast genome. The transgenic plants showed altered metabolism of C (lower leaf starch and soluble sugar accumulation) and N (with higher amounts of amino acids and soluble protein), which pointed to an activation of N metabolism at the expense of carbohydrates. To further delineate the effect of Trx m overexpression, metabolomic and enzymatic analyses were performed on these plants. These results showed an up-regulation of the glutamine synthetase-glutamate synthase pathway; specifically tobacco plants overexpressing Trx m displayed increased activity and stability of glutamine synthetase. Moreover, higher photorespiration and nitrate accumulation were observed in these plants relative to untransformed control plants, indicating that overexpression of Trx m favors the photorespiratory N cycle rather than primary nitrate assimilation. Taken together, our results reveal the importance of Trx m as a molecular mediator of N metabolism in plant chloroplasts.
Keywords: Carbon metabolism; GS-GOGAT pathway; chloroplast; glutamine synthetase; nitrogen metabolism; photorespiration; thioredoxin.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
-
- Aranjuelo I, Tcherkez G, Jauregui I, Gilard F, Ancín M, Fernández-San Millán A, Larraya L, Veramendi J, Farran I. 2015. Alteration by thioredoxin f over-expression of primary carbon metabolism and its response to elevated CO2 in tobacco (Nicotiana tabacum L.). Environmental and Experimental Botany 118, 40–48.
-
- Bénard C, Gibon Y. 2016. Measurement of enzyme activities and optimization of continuous and discontinuous assays. Current Protocols in Plant Biology 1, 247–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

