A 6-month inhalation toxicology study in Apoe-/- mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract
- PMID: 33963423
- PMCID: PMC8113187
- DOI: 10.1007/s00204-021-03020-4
A 6-month inhalation toxicology study in Apoe-/- mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract
Abstract
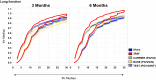
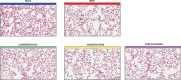
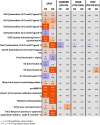
Cigarette smoking is the major cause of chronic obstructive pulmonary disease. Considerable attention has been paid to the reduced harm potential of nicotine-containing inhalable products such as electronic cigarettes (e-cigarettes). We investigated the effects of mainstream cigarette smoke (CS) and e-vapor aerosols (containing nicotine and flavor) generated by a capillary aerosol generator on emphysematous changes, lung function, and molecular alterations in the respiratory system of female Apoe-/- mice. Mice were exposed daily (3 h/day, 5 days/week) for 6 months to aerosols from three different e-vapor formulations-(1) carrier (propylene glycol and vegetable glycerol), (2) base (carrier and nicotine), or (3) test (base and flavor)-or to CS from 3R4F reference cigarettes. The CS and base/test aerosol concentrations were matched at 35 µg nicotine/L. CS exposure, but not e-vapor exposure, led to impairment of lung function (pressure-volume loop area, A and K parameters, quasi-static elastance and compliance) and caused marked lung inflammation and emphysematous changes, which were confirmed histopathologically and morphometrically. CS exposure caused lung transcriptome (activation of oxidative stress and inflammatory responses), lipidome, and proteome dysregulation and changes in DNA methylation; in contrast, these effects were substantially reduced in response to the e-vapor aerosol exposure. Compared with sham, aerosol exposure (carrier, base, and test) caused a slight impact on lung inflammation and epithelia irritation. Our results demonstrated that, in comparison with CS, e-vapor aerosols induced substantially lower biological and pathological changes in the respiratory tract associated with chronic inflammation and emphysema.
Keywords: COPD; Electronic cigarette; Emphysema; Inflammation; Smoking.
Conflict of interest statement
The testing facilities (Singapore and Neuchâtel) are owned by Philip Morris Products S.A. This work involved e-vapor formulations from Altria Client Services LLC (ALCS). All authors, except AB and WKS, are employees of ALCS or Philip Morris Products S.A. WKS is contracted and paid by Philip Morris Products S.A. AB is an employee of Histovia GmbH, which was contracted and paid by Philip Morris Products S.A to perform the histopathological analysis.
Figures


References
-
- Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR, Teeguarden JG. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol. 2014;26:829–842. doi: 10.3109/08958378.2014.935535. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

