Driveline exit-site care protocols in patients with left ventricular assist devices: a systematic review
- PMID: 33963835
- PMCID: PMC8434872
- DOI: 10.1093/ejcts/ezab195
Driveline exit-site care protocols in patients with left ventricular assist devices: a systematic review
Abstract
Objectives: Driveline infections continue to be a significant complication following left ventricular assist device (LVAD) implantation. Driveline exit-site care is crucial for the prevention of infections; however, there are no uniform guidelines. The goal of this study was to provide an overview of the currently published driveline exit-site care protocols in patients with LVAD.
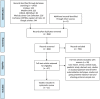
Methods: A systematic literature review was performed. Studies before 15 December 2020 were included if the number of driveline infections was a primary outcome and the driveline exit-site care protocol was explained.
Results: Eleven articles were included in the systematic review, including 1602 patients with LVADs. The median of the frequency of driveline infections in the articles was 13.8% with a range of 0-52.6%. There was a marked variability in the methods of care of driveline exit sites, without a standardized driveline dressing technique in patients with LVADs. The frequency of driveline infections was 6-7.5% in studies using a dressing kit that included chlorhexidine, a silver-based dressing and an anchoring device. Furthermore, there was variability in the anchoring devices and the frequency of dressing changes, which varied from daily to weekly. No specific anchoring device or change frequency was found to be superior.
Conclusions: Based on this systematic review, driveline exit care protocols that included chlorhexidine, a silver-based dressing, the use of an anchoring device and dressing kits might be best in reducing driveline infection rates. However, prospective studies with larger cohorts are needed to establish the optimal protocol for driveline exit-site care.
Keywords: Care; Dressing; Driveline; Driveline infection; Left ventricular assist device; Protocols.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures
References
-
- Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL. et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. - PubMed
-
- Hannan MM, Xie R, Cowger J, Schueler S, de By T, Dipchand AI. et al. Epidemiology of infection in mechanical circulatory support: a global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2019;38:364–73. - PubMed
-
- Goldstein DJ, Naftel D, Holman W, Bellumkonda L, Pamboukian SV, Pagani FD. et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 2012;31:1151–7. - PubMed
-
- Sharma V, Deo SV, Stulak JM, Durham LA 3rd, Daly RC, Park SJ. et al. Driveline infections in left ventricular assist devices: implications for destination therapy. Ann Thorac Surg 2012;94:1381–6. - PubMed
-
- Hieda M, Sata M, Seguchi O, Yanase M, Murata Y, Sato T. et al. Importance of early appropriate intervention including antibiotics and wound care for device-related infection in patients with left ventricular assist device. Transplant Proc 2014;46:907–10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical