Low-molecular-mass labile metal pools in Escherichia coli: advances using chromatography and mass spectrometry
- PMID: 33963934
- PMCID: PMC8205893
- DOI: 10.1007/s00775-021-01864-w
Low-molecular-mass labile metal pools in Escherichia coli: advances using chromatography and mass spectrometry
Abstract
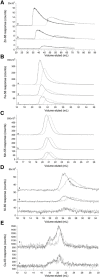
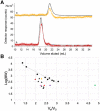
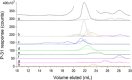
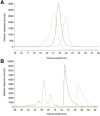
Labile low-molecular-mass (LMM) transition metal complexes play essential roles in metal ion trafficking, regulation, and signalling in biological systems, yet their chemical identities remain largely unknown due to their rapid ligand-exchange rates and weak M-L bonds. Here, an Escherichia coli cytosol isolation procedure was developed that was devoid of detergents, strongly coordinating buffers, and EDTA. The interaction of the metal ions from these complexes with a SEC column was minimized by pre-loading the column with 67ZnSO4 and then monitoring 66Zn and other metals by inductively coupled plasma mass spectrometry (ICP-MS) when investigating cytosolic ultrafiltration flow-through-solutions (FTSs). Endogenous cytosolic salts suppressed ESI-MS signals, making the detection of metal complexes difficult. FTSs contained ca. 80 µM Fe, 15 µM Ni, 13 µM Zn, 10 µM Cu, and 1.4 µM Mn (after correcting for dilution during cytosol isolation). FTSs exhibited 2-5 Fe, at least 2 Ni, 2-5 Zn, 2-4 Cu, and at least 2 Mn species with apparent masses between 300 and 5000 Da. Fe(ATP), Fe(GSH), and Zn(GSH) standards were passed through the column to assess their presence in FTS. Major LMM sulfur- and phosphorus-containing species were identified. These included reduced and oxidized glutathione, methionine, cysteine, orthophosphate, and common mono- and di-nucleotides such as ATP, ADP, AMP, and NADH. FTSs from cells grown in media supplemented with one of these metal salts exhibited increased peak intensity for the supplemented metal indicating that the size of the labile metal pools in E. coli is sensitive to the concentration of nutrient metals.
Keywords: Copper; Cytosol; Electrospray ionization mass spectrometry; Iron; Labile metal pools; Manganese; Size-exclusion chromatography; Zinc.
Conflict of interest statement
There are no conflicts or competing interests to declare.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

