Quantitative investigation of memory recall performance of a computational microcircuit model of the hippocampus
- PMID: 33963952
- PMCID: PMC8106564
- DOI: 10.1186/s40708-021-00131-7
Quantitative investigation of memory recall performance of a computational microcircuit model of the hippocampus
Abstract
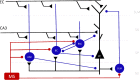
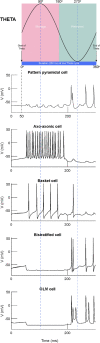
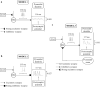
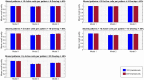
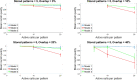
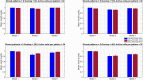
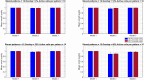
Memory, the process of encoding, storing, and maintaining information over time to influence future actions, is very important in our lives. Losing it, it comes with a great cost. Deciphering the biophysical mechanisms leading to recall improvement should thus be of outmost importance. In this study, we embarked on the quest to improve computationally the recall performance of a bio-inspired microcircuit model of the mammalian hippocampus, a brain region responsible for the storage and recall of short-term declarative memories. The model consisted of excitatory and inhibitory cells. The cell properties followed closely what is currently known from the experimental neurosciences. Cells' firing was timed to a theta oscillation paced by two distinct neuronal populations exhibiting highly regular bursting activity, one tightly coupled to the trough and the other to the peak of theta. An excitatory input provided to excitatory cells context and timing information for retrieval of previously stored memory patterns. Inhibition to excitatory cells acted as a non-specific global threshold machine that removed spurious activity during recall. To systematically evaluate the model's recall performance against stored patterns, pattern overlap, network size, and active cells per pattern, we selectively modulated feedforward and feedback excitatory and inhibitory pathways targeting specific excitatory and inhibitory cells. Of the different model variations (modulated pathways) tested, 'model 1' recall quality was excellent across all conditions. 'Model 2' recall was the worst. The number of 'active cells' representing a memory pattern was the determining factor in improving the model's recall performance regardless of the number of stored patterns and overlap between them. As 'active cells per pattern' decreased, the model's memory capacity increased, interference effects between stored patterns decreased, and recall quality improved.
Keywords: Bistratified cell; Computer model; Dendrite; Excitation; Inhibition; Medial septum; Memory retrieval; OLM cell; Pyramidal cell; Theta rhythm.
Conflict of interest statement
Authors declare they has no competing financial interests.
Figures








References
-
- Cutsuridis V, Graham BP, Cobb S, Vida I. Hippocampal microcircuits: a computational modeller’s resource book. 2. Cham: Springer; 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

