A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes
- PMID: 33964002
- PMCID: PMC8217049
- DOI: 10.1007/s40265-021-01499-w
A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes
Abstract
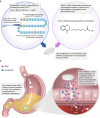
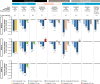
Oral semaglutide (Rybelsus®) is a glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA) with 94% homology to human GLP-1. It is the first GLP-1RA developed for oral administration, and it comprises a co-formulation of the peptide semaglutide with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl] amino) caprylate, which overcomes the challenges of peptide absorption in the acidic conditions of the stomach. Oral semaglutide is indicated for use as an add-on combination therapy (with other glucose-lowering agents, including insulin) or as a monotherapy (in patients who are intolerant to metformin) for type 2 diabetes when diet and exercise do not provide adequate glycemic control. In an extensive phase III clinical program including patients from across the disease spectrum, treatment with oral semaglutide resulted in effective glycemic control, reductions in body weight, and decreases in systolic blood pressure when used as monotherapy or in combination with other glucose-lowering therapies. Studies showed that oral semaglutide was well tolerated, with a safety profile consistent with the GLP-1RA drug class. The risk of hypoglycemia was low, and the most common adverse events were gastrointestinal, with nausea and diarrhea generally being the most frequently reported manifestations. Cardiovascular (CV) safety was shown to be noninferior to placebo and observations suggest that the CV profile of oral semaglutide is likely to be similar to that of subcutaneous semaglutide. The evolution of the GLP-1RA class to include an oral agent could facilitate the use of these agents earlier in the diabetes treatment cascade owing to wider acceptance from patients and healthcare professionals.
Conflict of interest statement
Andreas Andersen declares no conflicts of interest. Filip Krag Knop has served on scientific advisory panels and/or been part of speakers’ bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma. Tina Vilsbøll has served on scientific advisory panels, been part of speakers’ bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi, and Sun Pharmaceuticals.
Figures

References
-
- Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26(1):107–139. - PubMed
-
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

