Interdependencies between Toll-like receptors in Leishmania infection
- PMID: 33964011
- PMCID: PMC8358723
- DOI: 10.1111/imm.13364
Interdependencies between Toll-like receptors in Leishmania infection
Abstract
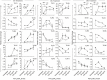
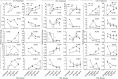
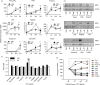
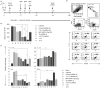
Multiple pathogen-associated molecular patterns (PAMPs) on a pathogen's surface imply their simultaneous recognition by the host cell membrane-located multiple PAMP-specific Toll-like receptors (TLRs). The TLRs on endosomes recognize internalized pathogen-derived nucleic acids and trigger anti-pathogen immune responses aimed at eliminating the intracellular pathogen. Whether the TLRs influence each other's expression and effector responses-termed TLR interdependency-remains unknown. Herein, we first probed the existence of TLR interdependencies and next determined how targeting TLR interdependencies might determine the outcome of Leishmania infection. We observed that TLRs selectively altered expression of their own and of other TLRs revealing novel TLR interdependencies. Leishmania major-an intra-macrophage parasite inflicting the disease cutaneous leishmaniasis in 88 countries-altered this TLR interdependency unfolding a unique immune evasion mechanism. We targeted this TLR interdependency by selective silencing of rationally chosen TLRs and by stimulation with selective TLR ligands working out a novel phase-specific treatment regimen. Targeting the TLR interdependency elicited a host-protective anti-leishmanial immune response and reduced parasite burden. To test whether this observation could be used as a scientific rationale for treating a potentially fatal L. donovani infection, which causes visceral leishmaniasis, we targeted the inter-TLR dependency adopting the same treatment regimen. We observed reduced splenic Leishman-Donovan units accompanied by host-protective immune response in susceptible BALB/c mice. The TLR interdependency optimizes TLR-induced immune response by a novel immunoregulatory framework and scientifically rationalizes targeting TLRs in tandem and in sequence for redirecting immune responses against an intracellular pathogen.
Keywords: Leishmania major; Toll-like receptors; cytokines; macrophages.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
Only CAG and TE have been named as co‐inventors of BPPcysMPEG. All other authors declare no conflict of interest.
Figures






References
-
- Anderson KV, Jürgens G, Nüsslein‐Volhard C. Establishment of dorsal‐ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–89. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/Cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. - PubMed
-
- Medzhitov R, Preston‐Hurlburt P, Janeway CA. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

