A Synthetic Galectin Mimic
- PMID: 33964110
- PMCID: PMC8361779
- DOI: 10.1002/anie.202104924
A Synthetic Galectin Mimic
Abstract
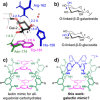
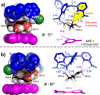
Galectins are a galactoside specific subclass of carbohydrate binding proteins (lectins) involved in various cellular activities, certain cancers, infections, inflammations, and many other biological processes. The molecular basis for the selectivity of galectins is well-documented and revolves around appropriate interaction complementarity: an aromatic residue for C-H⋅⋅⋅π interactions and polar residues for (charge assisted) hydrogen bonds with the axial hydroxyl group of a galactoside. However, no synthetic mimics are currently available. We now report on the design and synthesis of the first galectin mimic (6), and show that it has a higher than 65-fold preference for n-octyl-β-galactoside (8) over n-octyl-β-glucoside (7) in CD2 Cl2 containing 5 % [D6 ]DMSO (with Ka ≥4500 M-1 for 6:8). Molecular modeling informed by nOe studies reveal a high degree of interaction complementarity between 6 and galactoside 8, which is very similar to the interaction complementarity found in natural galectins.
Keywords: carbohydrate recognition; carbohydrates; galactosides; galectin mimic; molecular recognition.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition.J Biol Chem. 2006 Nov 24;281(47):35884-93. doi: 10.1074/jbc.M606648200. Epub 2006 Sep 21. J Biol Chem. 2006. PMID: 16990264
-
Structural analysis of the human galectin-9 N-terminal carbohydrate recognition domain reveals unexpected properties that differ from the mouse orthologue.J Mol Biol. 2008 Jan 4;375(1):119-35. doi: 10.1016/j.jmb.2007.09.060. Epub 2007 Sep 26. J Mol Biol. 2008. PMID: 18005988
-
Enzymatic synthesis of non-natural trisaccharides and galactosides; Insights of their interaction with galectins as a function of their structure.Carbohydr Res. 2019 Jan 15;472:1-15. doi: 10.1016/j.carres.2018.10.011. Epub 2018 Nov 3. Carbohydr Res. 2019. PMID: 30428394
-
Binding and cross-linking properties of galectins.Biochim Biophys Acta. 2002 Sep 19;1572(2-3):255-62. doi: 10.1016/s0304-4165(02)00312-4. Biochim Biophys Acta. 2002. PMID: 12223273 Review.
-
Oligosaccharide specificity of galectins: a search by frontal affinity chromatography.Biochim Biophys Acta. 2002 Sep 19;1572(2-3):232-54. doi: 10.1016/s0304-4165(02)00311-2. Biochim Biophys Acta. 2002. PMID: 12223272 Review.
Cited by
-
From small changes to big gains: pyridinium-based tetralactam macrocycle for enhanced sugar recognition in water.Chem Sci. 2024 Nov 5;15(46):19588-19598. doi: 10.1039/d4sc06190j. eCollection 2024 Nov 27. Chem Sci. 2024. PMID: 39568916 Free PMC article.
-
Anion binding properties of a hollow PdL-cage.Chem Commun (Camb). 2021 Jul 20;57(58):7184-7187. doi: 10.1039/d1cc02663a. Chem Commun (Camb). 2021. PMID: 34190254 Free PMC article.
-
Molecular Tweezers for Biomimetic Recognition of Carbohydrates.Chembiochem. 2025 Jun 3;26(11):e202500123. doi: 10.1002/cbic.202500123. Epub 2025 Mar 27. Chembiochem. 2025. PMID: 40146926 Free PMC article. Review.
-
Complexation-driven assembly of imine-linked helical receptors showing adaptive folding and temperature-dependent guest selection.Nat Commun. 2024 Feb 19;15(1):1501. doi: 10.1038/s41467-024-45322-y. Nat Commun. 2024. PMID: 38374171 Free PMC article.
-
Development and validation a nomogram prediction model for early diagnosis of bloodstream infections in the intensive care unit.Front Cell Infect Microbiol. 2024 Mar 4;14:1348896. doi: 10.3389/fcimb.2024.1348896. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38500500 Free PMC article.
References
-
- None
-
- Barondes S. H., Castronovo V., Cooper D. N. W., et al., Cell 1994, 76, 597–598; - PubMed
-
- Barondes S. H., Cooper D. N. W., Gitt M. A., et al., J. Biol. Chem. 1994, 269, 20807–20810; - PubMed
-
- Leffler H., Carlsson S., Hedlund M., et al., Glycoconjugate J. 2002, 19, 433–440; - PubMed
-
- Dumic J., Dabelic S., Flogel M., Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 616–635; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

