A Synthetic Galectin Mimic
- PMID: 33964110
- PMCID: PMC8361779
- DOI: 10.1002/anie.202104924
A Synthetic Galectin Mimic
Abstract
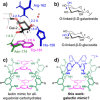
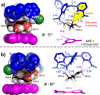
Galectins are a galactoside specific subclass of carbohydrate binding proteins (lectins) involved in various cellular activities, certain cancers, infections, inflammations, and many other biological processes. The molecular basis for the selectivity of galectins is well-documented and revolves around appropriate interaction complementarity: an aromatic residue for C-H⋅⋅⋅π interactions and polar residues for (charge assisted) hydrogen bonds with the axial hydroxyl group of a galactoside. However, no synthetic mimics are currently available. We now report on the design and synthesis of the first galectin mimic (6), and show that it has a higher than 65-fold preference for n-octyl-β-galactoside (8) over n-octyl-β-glucoside (7) in CD2 Cl2 containing 5 % [D6 ]DMSO (with Ka ≥4500 M-1 for 6:8). Molecular modeling informed by nOe studies reveal a high degree of interaction complementarity between 6 and galactoside 8, which is very similar to the interaction complementarity found in natural galectins.
Keywords: carbohydrate recognition; carbohydrates; galactosides; galectin mimic; molecular recognition.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Barondes S. H., Castronovo V., Cooper D. N. W., et al., Cell 1994, 76, 597–598; - PubMed
-
- Barondes S. H., Cooper D. N. W., Gitt M. A., et al., J. Biol. Chem. 1994, 269, 20807–20810; - PubMed
-
- Leffler H., Carlsson S., Hedlund M., et al., Glycoconjugate J. 2002, 19, 433–440; - PubMed
-
- Dumic J., Dabelic S., Flogel M., Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 616–635; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

