Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy
- PMID: 33964142
- PMCID: PMC8634121
- DOI: 10.1093/brain/awab184
Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy
Abstract
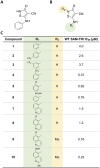
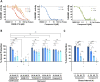
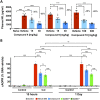
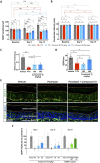
Axonal degeneration is an early and ongoing event that causes disability and disease progression in many neurodegenerative disorders of the peripheral and central nervous systems. Chemotherapy-induced peripheral neuropathy (CIPN) is a major cause of morbidity and the main cause of dose reductions and discontinuations in cancer treatment. Preclinical evidence indicates that activation of the Wallerian-like degeneration pathway driven by sterile alpha and TIR motif containing 1 (SARM1) is responsible for axonopathy in CIPN. SARM1 is the central driver of an evolutionarily conserved programme of axonal degeneration downstream of chemical, inflammatory, mechanical or metabolic insults to the axon. SARM1 contains an intrinsic NADase enzymatic activity essential for its pro-degenerative functions, making it a compelling therapeutic target to treat neurodegeneration characterized by axonopathies of the peripheral and central nervous systems. Small molecule SARM1 inhibitors have the potential to prevent axonal degeneration in peripheral and central axonopathies and to provide a transformational disease-modifying treatment for these disorders. Using a biochemical assay for SARM1 NADase we identified a novel series of potent and selective irreversible isothiazole inhibitors of SARM1 enzymatic activity that protected rodent and human axons in vitro. In sciatic nerve axotomy, we observed that these irreversible SARM1 inhibitors decreased a rise in nerve cADPR and plasma neurofilament light chain released from injured sciatic nerves in vivo. In a mouse paclitaxel model of CIPN we determined that Sarm1 knockout mice prevented loss of axonal function, assessed by sensory nerve action potential amplitudes of the tail nerve, in a gene-dosage-dependent manner. In that CIPN model, the irreversible SARM1 inhibitors prevented loss of intraepidermal nerve fibres induced by paclitaxel and provided partial protection of axonal function assessed by sensory nerve action potential amplitude and mechanical allodynia.
Keywords: ALS; CIPN; axonal degeneration; multiple sclerosis; neurodegeneration.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Cavanagh JB. The ‘dying back’ process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med. 1979;103(13):659–664. - PubMed
-
- Krauss R, Bosanac T, Devraj R, Engber T, Hughes RO.. Axons matter: The promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol Sci. 2020;41(4):281–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

