Vaccine Effectiveness Following Routine Immunization With Bivalent Human Papillomavirus (HPV) Vaccine: Protection Against Incident Genital HPV Infections From a Reduced-Dosing Schedule
- PMID: 33964158
- PMCID: PMC9441205
- DOI: 10.1093/infdis/jiab250
Vaccine Effectiveness Following Routine Immunization With Bivalent Human Papillomavirus (HPV) Vaccine: Protection Against Incident Genital HPV Infections From a Reduced-Dosing Schedule
Abstract
Background: In the Netherlands, the bivalent human papillomavirus (HPV) vaccine has been offered to preadolescent girls via the National Immunization Program in a 2-dose schedule since 2014. The current study estimates vaccine effectiveness (VE) against HPV infections up to 4 years postvaccination among girls eligible for routine 2-dose immunization.
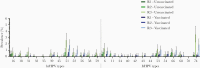
Methods: A cohort study (HAVANA2) was used in which participants annually filled out an online questionnaire and provided a vaginal self-sample for determination of HPV by the SPF10-LiPA25 assay, able to detect 25 HPV types. VE against incident type-specific infections and pooled outcomes was estimated by a Cox proportional hazards model with shared frailty between the HPV types.
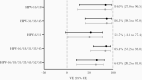
Results: In total, 2027 girls were included in the study, 1098 (54.2%) of whom were vaccinated with 2 doses. Highest incidence rate was 5.0/1000 person-years (HPV-51) among vaccinated participants and 9.1/1000 person-years (HPV-74) among unvaccinated participants. Adjusted pooled VE was 84.0% (95% confidence interval [CI], 27.0%-96.5%) against incident HPV-16/18 infections and 86.5% (95% CI, 39.5%-97.08%) against cross-protective types HPV-31/33/45.
Conclusions: Four years postvaccination, 2 doses of bivalent HPV vaccine were effective in the prevention of incident HPV-16/18 infections and provided cross-protection to HPV-31/33/45. Our VE estimates rival those from 3-dose schedules, indicating comparable protection by 2-dose schedules.
Keywords: human papillomavirus; immunization schedule; observational study; reduced dosing; vaccination.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


Similar articles
-
High Effectiveness of the Bivalent Human Papillomavirus (HPV) Vaccine Against Incident and Persistent HPV Infections up to 6 Years After Vaccination in Young Dutch Women.J Infect Dis. 2018 Apr 23;217(10):1579-1589. doi: 10.1093/infdis/jiy067. J Infect Dis. 2018. PMID: 29409034
-
Bivalent Vaccine Effectiveness Against Type-Specific HPV Positivity: Evidence for Cross-Protection Against Oncogenic Types Among Dutch STI Clinic Visitors.J Infect Dis. 2018 Jan 4;217(2):213-222. doi: 10.1093/infdis/jix582. J Infect Dis. 2018. PMID: 29140439 Free PMC article.
-
Effectiveness of bivalent HPV vaccination against genital HPV DNA-positivity of a catch-up campaign at age 13-16 years compared to routine vaccination at age 12 years: a biennial repeated cross-sectional study.BMC Med. 2024 Oct 15;22(1):469. doi: 10.1186/s12916-024-03686-4. BMC Med. 2024. PMID: 39407233 Free PMC article.
-
Less than 3 doses of the HPV vaccine - Review of efficacy against virological and disease end points.Hum Vaccin Immunother. 2016 Jun 2;12(6):1394-402. doi: 10.1080/21645515.2016.1146429. Epub 2016 Mar 2. Hum Vaccin Immunother. 2016. PMID: 26933961 Free PMC article. Review.
-
Human Papillomavirus Infection and Vaccination.J Pediatr Nurs. 2016 Mar-Apr;31(2):e155-66. doi: 10.1016/j.pedn.2015.10.005. Epub 2015 Nov 14. J Pediatr Nurs. 2016. PMID: 26586310 Review.
Cited by
-
Projected health and economic effects of nonavalent versus bivalent human papillomavirus vaccination in preadolescence in the Netherlands.BMC Med. 2025 Jun 9;23(1):339. doi: 10.1186/s12916-025-04170-3. BMC Med. 2025. PMID: 40484946 Free PMC article.
-
Immune response following a two-dose schedule of bivalent HPV vaccination among girls and boys.Front Immunol. 2024 Jan 26;15:1327770. doi: 10.3389/fimmu.2024.1327770. eCollection 2024. Front Immunol. 2024. PMID: 38343547 Free PMC article.
-
Human papillomavirus prevalence and vaccine effectiveness in young women in Germany, 2017/2018: results from a nationwide study.Front Public Health. 2023 Aug 31;11:1204101. doi: 10.3389/fpubh.2023.1204101. eCollection 2023. Front Public Health. 2023. PMID: 37719724 Free PMC article.
-
Optimizing the Harms and Benefits of Cervical Screening in a Partially Vaccinated Population in Ontario, Canada: A Modeling Study.Med Decis Making. 2025 Jul;45(5):545-556. doi: 10.1177/0272989X251332597. Epub 2025 Apr 22. Med Decis Making. 2025. PMID: 40260498 Free PMC article.
-
Human papillomavirus vaccine effectiveness by number of doses: Updated systematic review of data from national immunization programs.Vaccine. 2022 Sep 2;40(37):5413-5432. doi: 10.1016/j.vaccine.2022.06.065. Epub 2022 Aug 12. Vaccine. 2022. PMID: 35965239 Free PMC article.
References
-
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol 2005; 32:16–24. - PubMed
-
- Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006; 24:S52–61. - PubMed
-
- Committee for Medicinal Products for Human Use . Assessment report for Cervarix. London, UK: European Medicines Agency, 2013.
-
- Pedersen C, Petaja T, Strauss G, et al. ; HPV Vaccine Adolescent Study Investigators Network . Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

