Cochlear supporting cells require GAS2 for cytoskeletal architecture and hearing
- PMID: 33964205
- PMCID: PMC8137675
- DOI: 10.1016/j.devcel.2021.04.017
Cochlear supporting cells require GAS2 for cytoskeletal architecture and hearing
Abstract
In mammals, sound is detected by mechanosensory hair cells that are activated in response to vibrations at frequency-dependent positions along the cochlear duct. We demonstrate that inner ear supporting cells provide a structural framework for transmitting sound energy through the cochlear partition. Humans and mice with mutations in GAS2, encoding a cytoskeletal regulatory protein, exhibit hearing loss due to disorganization and destabilization of microtubule bundles in pillar and Deiters' cells, two types of inner ear supporting cells with unique cytoskeletal specializations. Failure to maintain microtubule bundle integrity reduced supporting cell stiffness, which in turn altered cochlear micromechanics in Gas2 mutants. Vibratory responses to sound were measured in cochleae from live mice, revealing defects in the propagation and amplification of the traveling wave in Gas2 mutants. We propose that the microtubule bundling activity of GAS2 imparts supporting cells with mechanical properties for transmitting sound energy through the cochlea.
Keywords: Gas2; cochlea; cochlear mechanics; cytoskeleton; hearing loss; microtubules; supporting cells.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
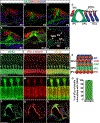

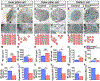

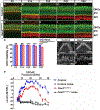


References
-
- Angelborg C and Engstrom H (1972). Supporting elements in the organ of Corti. I. Fibrillar structures in the supporting cells of the organ of Corti of mammals. Acta Otolaryngol. Suppl 73, 49–60. - PubMed
-
- Arima T, Uemura T, and Yamamoto T (1986). Cytoskeletal organization in the supporting cell of the guinea pig organ of Corti. Hear. Res. 24, 169–175. - PubMed
-
- Bekesy Gv. Experiments in Hearing. New York: McGraw-Hill, 1960.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

