Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial
- PMID: 33964223
- PMCID: PMC8121760
- DOI: 10.1016/S0140-6736(21)00943-0
Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial
Abstract
Background: Stalled progress in controlling Plasmodium falciparum malaria highlights the need for an effective and deployable vaccine. RTS,S/AS01, the most effective malaria vaccine candidate to date, demonstrated 56% efficacy over 12 months in African children. We therefore assessed a new candidate vaccine for safety and efficacy.
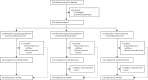
Methods: In this double-blind, randomised, controlled, phase 2b trial, the low-dose circumsporozoite protein-based vaccine R21, with two different doses of adjuvant Matrix-M (MM), was given to children aged 5-17 months in Nanoro, Burkina Faso-a highly seasonal malaria transmission setting. Three vaccinations were administered at 4-week intervals before the malaria season, with a fourth dose 1 year later. All vaccines were administered intramuscularly into the thigh. Group 1 received 5 μg R21 plus 25 μg MM, group 2 received 5 μg R21 plus 50 μg MM, and group 3, the control group, received rabies vaccinations. Children were randomly assigned (1:1:1) to groups 1-3. An independent statistician generated a random allocation list, using block randomisation with variable block sizes, which was used to assign participants. Participants, their families, and the local study team were all masked to group allocation. Only the pharmacists preparing the vaccine were unmasked to group allocation. Vaccine safety, immunogenicity, and efficacy were evaluated over 1 year. The primary objective assessed protective efficacy of R21 plus MM (R21/MM) from 14 days after the third vaccination to 6 months. Primary analyses of vaccine efficacy were based on a modified intention-to-treat population, which included all participants who received three vaccinations, allowing for inclusion of participants who received the wrong vaccine at any timepoint. This trial is registered with ClinicalTrials.gov, NCT03896724.
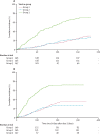
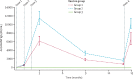
Findings: From May 7 to June 13, 2019, 498 children aged 5-17 months were screened, and 48 were excluded. 450 children were enrolled and received at least one vaccination. 150 children were allocated to group 1, 150 children were allocated to group 2, and 150 children were allocated to group 3. The final vaccination of the primary series was administered on Aug 7, 2019. R21/MM had a favourable safety profile and was well tolerated. The majority of adverse events were mild, with the most common event being fever. None of the seven serious adverse events were attributed to the vaccine. At the 6-month primary efficacy analysis, 43 (29%) of 146 participants in group 1, 38 (26%) of 146 participants in group 2, and 105 (71%) of 147 participants in group 3 developed clinical malaria. Vaccine efficacy was 74% (95% CI 63-82) in group 1 and 77% (67-84) in group 2 at 6 months. At 1 year, vaccine efficacy remained high, at 77% (67-84) in group 1. Participants vaccinated with R21/MM showed high titres of malaria-specific anti-Asn-Ala-Asn-Pro (NANP) antibodies 28 days after the third vaccination, which were almost doubled with the higher adjuvant dose. Titres waned but were boosted to levels similar to peak titres after the primary series of vaccinations after a fourth dose administered 1 year later.
Interpretation: R21/MM appears safe and very immunogenic in African children, and shows promising high-level efficacy.
Funding: The European & Developing Countries Clinical Trials Partnership, Wellcome Trust, and National Institute for Health Research Oxford Biomedical Research Centre.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AVSH and KJE are named as coinventors on patent applications related to R21. GG, LF, and JR are employees of Novavax, developers of the MM adjuvant, and US is an employee of the Serum Institute of India, codeveloper of the R21/MM vaccine. The other authors declare no competing interests.
Figures
Comment in
-
R21/Matrix-M: a second malaria vaccine?Lancet. 2021 May 15;397(10287):1782-1783. doi: 10.1016/S0140-6736(21)01065-5. Epub 2021 May 5. Lancet. 2021. PMID: 33964224 No abstract available.
References
-
- Gessner BD, Wraith DC, Finn A. CNS infection safety signal of RTS,S/AS01 and possible association with rabies vaccine. Lancet. 2016;387 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

