3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study
- PMID: 33964245
- PMCID: PMC8099316
- DOI: 10.1016/S2213-2600(21)00174-0
3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study
Abstract
Background: The consequences of COVID-19 in those who recover from acute infection requiring hospitalisation have yet to be clearly defined. We aimed to describe the temporal trends in respiratory outcomes over 12 months in patients hospitalised for severe COVID-19 and to investigate the associated risk factors.
Methods: In this prospective, longitudinal, cohort study, patients admitted to hospital for severe COVID-19 who did not require mechanical ventilation were prospectively followed up at 3 months, 6 months, 9 months, and 12 months after discharge from Renmin Hospital of Wuhan University, Wuhan, China. Patients with a history of hypertension; diabetes; cardiovascular disease; cancer; and chronic lung disease, including asthma or chronic obstructive pulmonary disease; or a history of smoking documented at time of hospital admission were excluded at time of electronic case-note review. Patients who required intubation and mechanical ventilation were excluded given the potential for the consequences of mechanical ventilation itself to influence the factors under investigation. During the follow-up visits, patients were interviewed and underwent physical examination, routine blood test, pulmonary function tests (ie, diffusing capacity of the lungs for carbon monoxide [DLCO]; forced expiratory flow between 25% and 75% of forced vital capacity [FVC]; functional residual capacity; FVC; FEV1; residual volume; total lung capacity; and vital capacity), chest high-resolution CT (HRCT), and 6-min walk distance test, as well as assessment using a modified Medical Research Council dyspnoea scale (mMRC).
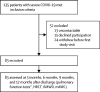
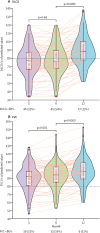
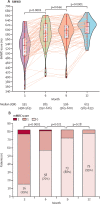
Findings: Between Feb 1, and March 31, 2020, of 135 eligible patients, 83 (61%) patients participated in this study. The median age of participants was 60 years (IQR 52-66). Temporal improvement in pulmonary physiology and exercise capacity was observed in most patients; however, persistent physiological and radiographic abnormalities remained in some patients with COVID-19 at 12 months after discharge. We found a significant reduction in DLCO over the study period, with a median of 77% of predicted (IQR 67-87) at 3 months, 76% of predicted (68-90) at 6 months, and 88% of predicted (78-101) at 12 months after discharge. At 12 months after discharge, radiological changes persisted in 20 (24%) patients. Multivariate logistic regression showed increasing odds of impaired DLCO associated with female sex (odds ratio 8·61 [95% CI 2·83-26·2; p=0·0002) and radiological abnormalities were associated with peak HRCT pneumonia scores during hospitalisation (1·36 [1·13-1·62]; p=0·0009).
Interpretation: In most patients who recovered from severe COVID-19, dyspnoea scores and exercise capacity improved over time; however, in a subgroup of patients at 12 months we found evidence of persistent physiological and radiographic change. A unified pathway for the respiratory follow-up of patients with COVID-19 is required.
Funding: National Natural Science Foundation of China, UK Medical Research Council, and National Institute for Health Research Southampton Biomedical Research Centre.
Translation: For the Chinese translation of the abstract see Supplementary Materials section.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
An integrated understanding of long-term sequelae after acute COVID-19.Lancet Respir Med. 2021 Jul;9(7):679-680. doi: 10.1016/S2213-2600(21)00206-X. Epub 2021 May 5. Lancet Respir Med. 2021. PMID: 33964246 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

