Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines?
- PMID: 33964339
- PMCID: PMC8099545
- DOI: 10.1016/j.ijpharm.2021.120686
Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines?
Abstract
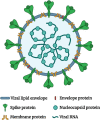
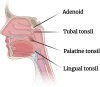
It is striking that all marketed SARS-CoV-2 vaccines are developed for intramuscular administration designed to produce humoral and cell mediated immune responses, preventing viremia and the COVID-19 syndrome. They have a high degree of efficacy in humans (70-95%) depending on the type of vaccine. However, little protection is provided against viral replication and shedding in the upper airways due to the lack of a local sIgA immune response, indicating a risk of transmission of virus from vaccinated individuals. A range of novel nasal COVID-19 vaccines are in development and preclinical results in non-human primates have shown a promising prevention of replication and shedding of virus due to the induction of mucosal immune response (sIgA) in upper and lower respiratory tracts as well as robust systemic and humoral immune responses. Whether these results will translate to humans remains to be clarified. An IM prime followed by an IN booster vaccination would likely result in a better well-rounded immune response, including prevention (or strong reduction) in viral replication in the upper and lower respiratory tracts.
Keywords: COVID-19; COVID-19 vaccines; Intramuscular COVID-19 vaccines; Intranasal COVID-19 vaccines; SARS-CoV-2; Vaccine immune responses.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Experts With One Big Claim: The Coronavirus Is Airborne - The New York Times [WWW Document]. URL https://www.nytimes.com/2020/07/04/health/239-experts-with-one-big-claim... (accessed 3.16.21).
-
- Allen, A.C., Mills, K.H.G., 2014. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev. Vaccines. DOI: 10.1586/14760584.2014.936391. - PubMed
-
- Altimmune Commences Enrollment in Phase 1 Clinical Trial of AdCOVIDTM -- a Needle-Free, Single-Dose Intranasal COVID-19 Vaccine Candidate – Altimmune [WWW Document]. URL https://ir.altimmune.com/news-releases/news-release-details/altimmune-co... (accessed 3.22.21).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous