Developing a database of systematic reviews of animal studies
- PMID: 33964349
- PMCID: PMC11364211
- DOI: 10.1016/j.yrtph.2021.104940
Developing a database of systematic reviews of animal studies
Abstract
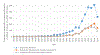
Systematic reviews (SRs) are common practice in clinical and public health research, but less common in non-human animal research. Systematic reviews of animal studies can be valuable to inform clinical research, to evaluate the need for further animal experiments on a given topic, and to assess the hazard of an environmental exposure in the evaluation of toxicological studies. In the last 10 years, there has been an increase in the number of SRs of animal research, as well as several publications with detailed guidance on how to perform high-quality systematic reviews of experimental animal studies. In order to evaluate current analytical approaches used in SRs of animal studies, easily identify all systematic reviews on a specific topic, and subsequently the original animal studies and their results and promote awareness and understanding of these emerging approaches, we compiled a database of SRs of animal studies. The database was developed using a rigorous, systematic approach and covers a broad range of research fields: preclinical research, toxicology, environmental health, and veterinary medicine. The database currently includes 3113 SRs of animal studies (search date June 2019). In addition to bibliographical information, data on whether or not a risk of bias assessment and meta-analysis were conducted were extracted. For future users, the search features of the database provide users with a platform to identify and select SRs with a particular characteristic for export to Microsoft Word or Microsoft Excel. From there, users may perform additional data extraction to meet their research needs. The database is freely available at www.Mendeley.com (link). The database provides methodologists a comprehensive source that can be used to explore and advance the current methodology applied to SRs of animal studies, and can help researchers to easily identify all systematic reviews on a specific topic, and subsequently the original animal studies and their results and avoid duplication and unnecessary animal research.
Keywords: Animal studies; Database; Systematic reviews.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Cochrane. Cochrane-REWARD prizes for reducing waste: 2017 winners. Available from: http://www.cochrane.org/news/cochrane-reward-prizes-reducing-waste-2017-....
-
- Craig JC, Wheeler DM, Irwig L, Howman-Giles RB, 2000. How accurate is dimercaptosuccinic acid scintigraphy for the diagnosis of acute pyelonephritis? A meta-analysis of experimental studies. J. Nucl. Med: official publication, Society of Nuclear Medicine; 41 (6), 986–993. - PubMed
-
- Freedman LS, 1994. Meta-analysis of animal experiments on dietary fat intake and mammary tumours. Stat. Med 13 (5–7), 709–718. - PubMed
-
- Haddaway NRMB, Whaley P, Pullin A, 2018. ROSES RepOrting standards for Systematic Evidence Syntheses: pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ. Evid 7 (7).
-
- Hebert PC, Hu LQ, Biro GP, 1997. Review of physiologic mechanisms in response to anemia. CMAJ (Can. Med. Assoc. J.): Can. Med. Assoc. J 156 (11), S27–S40.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

