Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds
- PMID: 33964480
- PMCID: PMC8273127
- DOI: 10.1016/j.actbio.2021.04.048
Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds
Abstract
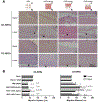
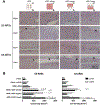
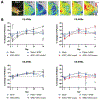
Vascularization is a critical step following implantation of an engineered tissue construct in order to maintain its viability. The ability to spatially pattern or direct vascularization could be therapeutically beneficial for anastomosis and vessel in-growth. However, acellular and cell-based strategies to stimulate vascularization typically do not afford this control. We have developed an ultrasound-based method of spatially- controlling regenerative processes using acellular, composite hydrogels termed acoustically-responsive scaffolds (ARSs). An ARS consists of a fibrin matrix doped with a phase-shift double emulsion (PSDE). A therapeutic payload, which is initially contained within the PSDE, is released by an ultrasound-mediated process called acoustic droplet vaporization (ADV). During ADV, the perfluorocarbon (PFC) phase within the PSDE is vaporized into a gas bubble. In this study, we generated ex situ four different spatial patterns of ADV within ARSs containing basic fibroblast growth factor (bFGF), which were subcutaneously implanted in mice. The PFC species within the PSDE significantly affected the morphology of the ARS, based on the stability of the gas bubble generated by ADV, which impacted host cell migration. Irrespective of PFC, significantly greater cell proliferation (i.e., up to 2.9-fold) and angiogenesis (i.e., up to 3.7-fold) were observed adjacent to +ADV regions of the ARSs compared to -ADV regions. The morphology of the PSDE, macrophage infiltration, and perfusion in the implant region were also quantified. These results demonstrate that spatially-defined patterns of ADV within an ARS can elicit spatially-defined patterns of angiogenesis. Overall, these finding can be applied to improve strategies for spatially-controlling vascularization. STATEMENT OF SIGNIFICANCE: Vascularization is a critical step following implantation of an engineered tissue. The ability to spatially pattern or direct vascularization could be therapeutically beneficial for inosculation and vessel in-growth. However, acellular and cell-based strategies to stimulate vascularization typically do not afford this control. We have developed an ultrasound-based method of spatially-controlling angiogenesis using acellular, composite hydrogels termed acoustically-responsive scaffolds (ARSs). An ARS consists of a fibrin matrix doped with a phase-shift double emulsion (PSDE). An ultrasound-mediated process called acoustic droplet vaporization (ADV) was used to release basic fibroblast growth factor (bFGF), which was initially contained within the PSDE. We demonstrate that spatially-defined patterns of ADV within an ARS can elicit spatially-defined patterns of angiogenesis in vivo. Overall, these finding can improve strategies for spatially-controlling vascularization.
Keywords: Acoustic droplet vaporization; Angiogenesis; Basic fibroblast growth factor; Drug delivery; Fibrin; Ultrasound.
Copyright © 2021 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Spatially-directed cell migration in acoustically-responsive scaffolds through the controlled delivery of basic fibroblast growth factor.Acta Biomater. 2020 Sep 1;113:217-227. doi: 10.1016/j.actbio.2020.06.015. Epub 2020 Jun 14. Acta Biomater. 2020. PMID: 32553916 Free PMC article.
-
Spatiotemporal control of micromechanics and microstructure in acoustically-responsive scaffolds using acoustic droplet vaporization.Soft Matter. 2020 Jul 22;16(28):6501-6513. doi: 10.1039/d0sm00753f. Soft Matter. 2020. PMID: 32597450 Free PMC article.
-
Acoustic droplet vaporization for on-demand modulation of microporosity in smart hydrogels.Acta Biomater. 2023 Jul 1;164:195-208. doi: 10.1016/j.actbio.2023.04.037. Epub 2023 Apr 29. Acta Biomater. 2023. PMID: 37121372 Free PMC article.
-
Bubble nucleation and dynamics in acoustic droplet vaporization: a review of concepts, applications, and new directions.Z Med Phys. 2023 Aug;33(3):387-406. doi: 10.1016/j.zemedi.2023.01.004. Epub 2023 Feb 10. Z Med Phys. 2023. PMID: 36775778 Free PMC article. Review.
-
Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis.Curr Pharm Des. 2007;13(35):3597-607. doi: 10.2174/138161207782794158. Curr Pharm Des. 2007. PMID: 18220797 Review.
Cited by
-
Current research trends of nanomedicines.Acta Pharm Sin B. 2023 Nov;13(11):4391-4416. doi: 10.1016/j.apsb.2023.05.018. Epub 2023 May 20. Acta Pharm Sin B. 2023. PMID: 37969727 Free PMC article. Review.
-
In vivo acoustic patterning of endothelial cells for tissue vascularization.Sci Rep. 2023 Sep 26;13(1):16082. doi: 10.1038/s41598-023-43299-0. Sci Rep. 2023. PMID: 37752255 Free PMC article.
-
Emerging Bioactive Agent Delivery-Based Regenerative Therapies for Lower Genitourinary Tissues.Pharmaceutics. 2022 Aug 17;14(8):1718. doi: 10.3390/pharmaceutics14081718. Pharmaceutics. 2022. PMID: 36015344 Free PMC article. Review.
-
Real-time spatiotemporal characterization of mechanics and sonoporation of acoustic droplet vaporization in acoustically responsive scaffolds.Appl Phys Lett. 2023 Sep 11;123(11):114101. doi: 10.1063/5.0159661. Appl Phys Lett. 2023. PMID: 37705893 Free PMC article.
-
Ultrasound-Induced Drug Release from Stimuli-Responsive Hydrogels.Gels. 2022 Sep 1;8(9):554. doi: 10.3390/gels8090554. Gels. 2022. PMID: 36135267 Free PMC article. Review.
References
-
- Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE, Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration, Journal of cell science 116(Pt 14) (2003) 2917–27. - PubMed
-
- Zarem HA, The microcirculatory events within full-thickness skin allografts (homografts) in mice, Surgery 66(2) (1969) 392–7. - PubMed
-
- Chung YI, Kim SK, Lee YK, Park SJ, Cho KO, Yuk SH, Tae G, Kim YH, Efficient revascularization by VEGF administration via heparin-functionalized nanoparticle-fibrin complex, Journal of Controlled Release 143(3) (2010) 282–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

