Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds
- PMID: 33964480
- PMCID: PMC8273127
- DOI: 10.1016/j.actbio.2021.04.048
Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds
Abstract
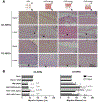
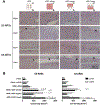
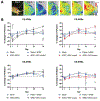
Vascularization is a critical step following implantation of an engineered tissue construct in order to maintain its viability. The ability to spatially pattern or direct vascularization could be therapeutically beneficial for anastomosis and vessel in-growth. However, acellular and cell-based strategies to stimulate vascularization typically do not afford this control. We have developed an ultrasound-based method of spatially- controlling regenerative processes using acellular, composite hydrogels termed acoustically-responsive scaffolds (ARSs). An ARS consists of a fibrin matrix doped with a phase-shift double emulsion (PSDE). A therapeutic payload, which is initially contained within the PSDE, is released by an ultrasound-mediated process called acoustic droplet vaporization (ADV). During ADV, the perfluorocarbon (PFC) phase within the PSDE is vaporized into a gas bubble. In this study, we generated ex situ four different spatial patterns of ADV within ARSs containing basic fibroblast growth factor (bFGF), which were subcutaneously implanted in mice. The PFC species within the PSDE significantly affected the morphology of the ARS, based on the stability of the gas bubble generated by ADV, which impacted host cell migration. Irrespective of PFC, significantly greater cell proliferation (i.e., up to 2.9-fold) and angiogenesis (i.e., up to 3.7-fold) were observed adjacent to +ADV regions of the ARSs compared to -ADV regions. The morphology of the PSDE, macrophage infiltration, and perfusion in the implant region were also quantified. These results demonstrate that spatially-defined patterns of ADV within an ARS can elicit spatially-defined patterns of angiogenesis. Overall, these finding can be applied to improve strategies for spatially-controlling vascularization. STATEMENT OF SIGNIFICANCE: Vascularization is a critical step following implantation of an engineered tissue. The ability to spatially pattern or direct vascularization could be therapeutically beneficial for inosculation and vessel in-growth. However, acellular and cell-based strategies to stimulate vascularization typically do not afford this control. We have developed an ultrasound-based method of spatially-controlling angiogenesis using acellular, composite hydrogels termed acoustically-responsive scaffolds (ARSs). An ARS consists of a fibrin matrix doped with a phase-shift double emulsion (PSDE). An ultrasound-mediated process called acoustic droplet vaporization (ADV) was used to release basic fibroblast growth factor (bFGF), which was initially contained within the PSDE. We demonstrate that spatially-defined patterns of ADV within an ARS can elicit spatially-defined patterns of angiogenesis in vivo. Overall, these finding can improve strategies for spatially-controlling vascularization.
Keywords: Acoustic droplet vaporization; Angiogenesis; Basic fibroblast growth factor; Drug delivery; Fibrin; Ultrasound.
Copyright © 2021 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE, Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration, Journal of cell science 116(Pt 14) (2003) 2917–27. - PubMed
-
- Zarem HA, The microcirculatory events within full-thickness skin allografts (homografts) in mice, Surgery 66(2) (1969) 392–7. - PubMed
-
- Chung YI, Kim SK, Lee YK, Park SJ, Cho KO, Yuk SH, Tae G, Kim YH, Efficient revascularization by VEGF administration via heparin-functionalized nanoparticle-fibrin complex, Journal of Controlled Release 143(3) (2010) 282–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

