Follistatin-induced muscle hypertrophy in aged mice improves neuromuscular junction innervation and function
- PMID: 33964607
- PMCID: PMC8225567
- DOI: 10.1016/j.neurobiolaging.2021.03.005
Follistatin-induced muscle hypertrophy in aged mice improves neuromuscular junction innervation and function
Abstract
Sarcopenia, or age-related loss of muscle mass and strength, is an important contributor to loss of physical function in older adults. The pathogenesis of sarcopenia is likely multifactorial, but recently the role of neurological degeneration, such as motor unit loss, has received increased attention. Here, we investigated the longitudinal effects of muscle hypertrophy (via overexpression of human follistatin, a myostatin antagonist) on neuromuscular integrity in C57BL/6J mice between the ages of 24 and 27 months. Following follistatin overexpression (delivered via self-complementary adeno-associated virus subtype 9 injection), muscle weight and torque production were significantly improved. Follistatin treatment resulted in improvements of neuromuscular junction innervation and transmission but had no impact on age-related losses of motor units. These studies demonstrate that follistatin overexpression-induced muscle hypertrophy not only increased muscle weight and torque production but also countered age-related degeneration at the neuromuscular junction in mice.
Keywords: Adeno-associated; Contractility; Follistatin; Motor unit number estimate; Myostatin; Neuromuscular junction; Sarcopenia; Single fiber electromyography; Tetanic; Twitch.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Disclosure statement The authors have no conflicts of interests to report.
Figures


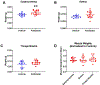


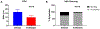

Similar articles
-
Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems.Ageing Res Rev. 2023 Aug;89:101966. doi: 10.1016/j.arr.2023.101966. Epub 2023 Jun 1. Ageing Res Rev. 2023. PMID: 37270145 Free PMC article. Review.
-
Voluntary wheel running with and without follistatin overexpression improves NMJ transmission but not motor unit loss in late life of C57BL/6J mice.Neurobiol Aging. 2021 May;101:285-296. doi: 10.1016/j.neurobiolaging.2021.01.012. Epub 2021 Feb 5. Neurobiol Aging. 2021. PMID: 33678425 Free PMC article.
-
Neuromuscular junction transmission failure is a late phenotype in aging mice.Neurobiol Aging. 2020 Feb;86:182-190. doi: 10.1016/j.neurobiolaging.2019.10.022. Epub 2019 Nov 5. Neurobiol Aging. 2020. PMID: 31866157 Free PMC article.
-
Effects of the activin A-myostatin-follistatin system on aging bone and muscle progenitor cells.Exp Gerontol. 2013 Feb;48(2):290-7. doi: 10.1016/j.exger.2012.11.004. Epub 2012 Nov 21. Exp Gerontol. 2013. PMID: 23178301 Free PMC article.
-
Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease.Muscle Nerve. 2009 Mar;39(3):283-96. doi: 10.1002/mus.21244. Muscle Nerve. 2009. PMID: 19208403 Free PMC article. Review.
Cited by
-
Cancer cachexia: molecular mechanisms and treatment strategies.J Hematol Oncol. 2023 May 22;16(1):54. doi: 10.1186/s13045-023-01454-0. J Hematol Oncol. 2023. PMID: 37217930 Free PMC article. Review.
-
Exploring motor unit and neuromuscular junction dysfunction in aging and sarcopenia: insights from electromyography in systematic review.Geroscience. 2025 Jul 8. doi: 10.1007/s11357-025-01760-0. Online ahead of print. Geroscience. 2025. PMID: 40627125 Review.
-
Myostatin Knockout Mice Have Larger Muscle Fibers With Normal Function and Morphology.Muscle Nerve. 2025 Jun;71(6):1122-1131. doi: 10.1002/mus.28389. Epub 2025 Mar 3. Muscle Nerve. 2025. PMID: 40026240 Free PMC article.
-
Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems.Ageing Res Rev. 2023 Aug;89:101966. doi: 10.1016/j.arr.2023.101966. Epub 2023 Jun 1. Ageing Res Rev. 2023. PMID: 37270145 Free PMC article. Review.
-
New intranasal and injectable gene therapy for healthy life extension.Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2121499119. doi: 10.1073/pnas.2121499119. Epub 2022 May 10. Proc Natl Acad Sci U S A. 2022. Retraction in: Proc Natl Acad Sci U S A. 2025 Aug 19;122(33):e2519570122. doi: 10.1073/pnas.2519570122. PMID: 35537048 Free PMC article. Retracted.
References
-
- Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbóva G, Partridge T, Zammit P, Bunger L, Patel K, 2007. Lack of myostatin results in excessive muscle growth but impaired force generation. Proceedings of the National Academy of Sciences 104(6), 1835–1840. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

