Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels
- PMID: 33964910
- PMCID: PMC8106850
- DOI: 10.1186/s12931-021-01734-8
Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels
Abstract
Background: We previously reported a Phase 1/2 randomized placebo-controlled trial of systemic administration of bone marrow-derived allogeneic MSCs (remestemcel-L) in COPD. While safety profile was good, no functional efficacy was observed. However, in view of growing recognition of effects of inflammatory environments on MSC actions we conducted a post-hoc analysis with stratification by baseline levels of a circulating inflammatory marker, C-reactive protein (CRP) to determine the effects of MSC administration in COPD patients with varying circulating CRP levels.
Methods: Time course of lung function, exercise performance, patient reported responses, and exacerbation frequency following four monthly infusions of remestemcel-L vs. placebo were re-assessed in subgroups based on baseline circulating CRP levels.
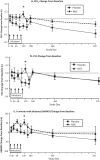
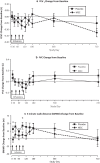
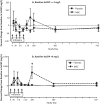
Results: In COPD patients with baseline CRP ≥ 4 mg/L, compared to COPD patients receiving placebo (N = 17), those treated with remestemcel-L (N = 12), demonstrated significant improvements from baseline in forced expiratory volume in one second, forced vital capacity, and six minute walk distance at 120 days with treatment differences evident as early as 10 days after the first infusion. Significant although smaller benefits were also detected in those with CRP levels ≥ 2 or ≥ 3 mg/L. These improvements persisted variably over the 2-year observational period. No significant benefits were observed in patient reported responses or number of COPD exacerbations between treatment groups.
Conclusion: In an inflammatory environment, defined by elevated circulating CRP, remestemcel-L administration yielded at least transient meaningful pulmonary and functional improvements. These findings warrant further investigation of potential MSC-based therapies in COPD and other inflammatory pulmonary diseases.
Trial registration: Clinicaltrials.gov NCT00683722.
Keywords: C-reactive protein; Chronic obstructive pulmonary disease; Inflammation; Mesenchymal stromal cells; Pulmonary function.
Conflict of interest statement
DJW receives research support from the NIH, Department of Defense, Cystic Fibrosis Foundation, and the University of Vermont. RC is a member of the speaker’s bureau for Glaxo Smith Kline, is a consultant for Genentech, Regeneron and Boehringer Ingelheim and is an advisory board member for Astra Zeneca. His laboratory receives research support from Regeneron, Genentech, Boehringer Ingelheim and Glaxo Smith Kline. KS is a former employee of Mesoblast Inc. and JH is a current employee of Mesoblast Inc. DPT is an advisory board member and speaker for AstraZeneca and Mylan/Theravance. DJW, RC, and DPT had previous research support from Osiris Therapeutics, Inc.
Figures
Similar articles
-
A placebo-controlled, randomized trial of mesenchymal stem cells in COPD.Chest. 2013 Jun;143(6):1590-1598. doi: 10.1378/chest.12-2094. Chest. 2013. PMID: 23172272 Free PMC article. Clinical Trial.
-
Elevated circulating PAI-1 levels are related to lung function decline, systemic inflammation, and small airway obstruction in chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2016 Sep 26;11:2369-2376. doi: 10.2147/COPD.S107409. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27713627 Free PMC article.
-
Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease.JAMA. 2013 Jun 12;309(22):2353-61. doi: 10.1001/jama.2013.5732. JAMA. 2013. PMID: 23757083
-
Mesenchymal stromal cell therapy in COPD: from bench to bedside.Int J Chron Obstruct Pulmon Dis. 2017 Oct 16;12:3017-3027. doi: 10.2147/COPD.S146671. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29081655 Free PMC article. Review.
-
Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease Using Commercial Mesenchymal Stem Cell Products.Front Immunol. 2021 Aug 19;12:724380. doi: 10.3389/fimmu.2021.724380. eCollection 2021. Front Immunol. 2021. PMID: 34489977 Free PMC article. Review.
Cited by
-
Mapping global trends in research of stem cell therapy for COVID-19: A bibliometric analysis.Front Public Health. 2022 Oct 14;10:1016237. doi: 10.3389/fpubh.2022.1016237. eCollection 2022. Front Public Health. 2022. PMID: 36311582 Free PMC article.
-
Paracrine Regulation of Alveolar Epithelial Damage and Repair Responses by Human Lung-Resident Mesenchymal Stromal Cells.Cells. 2021 Oct 23;10(11):2860. doi: 10.3390/cells10112860. Cells. 2021. PMID: 34831082 Free PMC article.
-
Transcriptional profiling of circulating mononuclear cells from patients with chronic obstructive pulmonary disease receiving mesenchymal stromal cell infusions.Stem Cells Transl Med. 2021 Nov;10(11):1470-1481. doi: 10.1002/sctm.21-0024. Epub 2021 Aug 18. Stem Cells Transl Med. 2021. PMID: 34405962 Free PMC article.
-
The Inflammatory Lung Microenvironment; a Key Mediator in MSC Licensing.Cells. 2021 Nov 2;10(11):2982. doi: 10.3390/cells10112982. Cells. 2021. PMID: 34831203 Free PMC article. Review.
-
Effects of secretome supplementation on interleukin-6, tumor necrosis factor-α, procalcitonin, and the length of stay in acute exacerbation COPD patients.Narra J. 2023 Aug;3(2):e171. doi: 10.52225/narra.v3i2.171. Epub 2023 Aug 9. Narra J. 2023. PMID: 38450275 Free PMC article.
References
-
- GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

