Investigation of clinically acceptable agreement between two methods of automatic measurement of limb occlusion pressure: a randomised trial
- PMID: 33964963
- PMCID: PMC8105974
- DOI: 10.1186/s42490-021-00053-9
Investigation of clinically acceptable agreement between two methods of automatic measurement of limb occlusion pressure: a randomised trial
Abstract
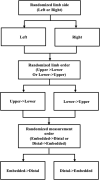
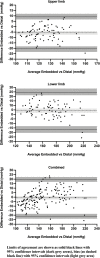
Background: Development of automatic, pneumatic tourniquet technology and use of personalised tourniquet pressures has improved the safety and accuracy of surgical tourniquet systems. Personalisation of tourniquet pressure requires accurate measurement of limb occlusion pressure (LOP), which can be measured automatically through two different methods. The 'embedded LOP' method measures LOP using a dual-purpose tourniquet cuff acting as both patient sensor and pneumatic effector. The 'distal LOP' method measures LOP using a distal sensor applied to the patient's finger or toe of the operating limb, using photoplethysmography to detect volumetric changes in peripheral blood circulation. The distal LOP method has been used clinically for many years; the embedded LOP method was developed recently with several advantages over the distal LOP method. While both methods have clinically acceptable accuracy in comparison to LOP measured using the manual Doppler ultrasound method, these two automatic methods have not been directly compared. The purpose of this study is to investigate if the embedded and distal methods of LOP measurement have clinically acceptable agreement. The differences in pairs of LOP measurement in the upper and lower limbs of 81 healthy individuals were compared using modified Bland and Altman analysis. In surgery, it is common for cuff pressure to deviate from the pressure setpoint due to limb manipulation. Surgical tourniquet systems utilise a ± 15 mmHg pressure alarm window, whereby if the cuff pressure deviates from the pressure setpoint by > 15 mmHg, an audiovisual alarm is triggered. Therefore, if the difference (bias) ± SE, 95% CI of the bias and SD of differences ± SE in LOP measurement between the embedded and distal methods were all within ±15 mmHg, this would demonstrate that the two methods have clinically acceptable agreement.
Results: LOP measurement using the embedded LOP method was - 0.81 ± 0.75 mmHg (bias ± standard error) lower than the distal LOP method. The 95% confidence interval of the bias was - 2.29 to 0.66 mmHg. The standard deviation of the differences ± standard error was 10.35 ± 0.49 mmHg. These results show that the embedded and distal methods of LOP measurement demonstrate clinically acceptable agreement.
Conclusions: The findings of this study demonstrate clinically acceptable agreement between the embedded and distal methods of LOP measurement. The findings support the use of the embedded LOP method of automatic LOP measurement using dual-purpose tourniquet cuffs to enable accurate, effective and simple prescription of personalised tourniquet cuff pressures in a clinical setting.
Keywords: Limb occlusion pressure; Personalised; Tourniquet; Tourniquet safety.
Conflict of interest statement
LH declares no competing or conflicting interests. JM is president and an indirect shareholder of Western Clinical Engineering Ltd., and has patents US 9,039,730, US 9,814,467, US 8,425,551 and US 20170112504 assigned to Western Clinical Engineering Ltd.
Figures
Similar articles
-
Technique for Measuring Limb Occlusion Pressure that Facilitates Personalized Tourniquet Systems: A Randomized Trial.J Med Biol Eng. 2016;36(5):644-650. doi: 10.1007/s40846-016-0173-5. Epub 2016 Oct 4. J Med Biol Eng. 2016. PMID: 27853415 Free PMC article.
-
Tourniquet safety in lower leg applications.Orthop Nurs. 2002 Sep-Oct;21(5):55-62. doi: 10.1097/00006416-200209000-00009. Orthop Nurs. 2002. PMID: 12432700 Clinical Trial.
-
Minimizing tourniquet pressure in pediatric anterior cruciate ligament reconstructive surgery: a blinded, prospective randomized controlled trial.J Pediatr Orthop. 2009 Apr-May;29(3):275-80. doi: 10.1097/BPO.0b013e31819bcd14. J Pediatr Orthop. 2009. PMID: 19305279 Clinical Trial.
-
Tourniquet-induced neuromuscular injury. A recent review of rabbit and clinical experiments.Acta Orthop Scand Suppl. 1991;245:1-33. Acta Orthop Scand Suppl. 1991. PMID: 1950503 Review.
-
A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery.J Clin Med. 2025 Mar 13;14(6):1938. doi: 10.3390/jcm14061938. J Clin Med. 2025. PMID: 40142747 Free PMC article. Review.
Cited by
-
Perioperative Arthroscopic Tourniquet Usage: Practices and Trends of the AANA Membership Are Varied.Arthrosc Sports Med Rehabil. 2025 Feb 20;7(3):101106. doi: 10.1016/j.asmr.2025.101106. eCollection 2025 Jun. Arthrosc Sports Med Rehabil. 2025. PMID: 40692915 Free PMC article.
-
Development and psychometric evaluation of a pneumatic tourniquet work standards scale.J Orthop Surg Res. 2024 Jul 26;19(1):433. doi: 10.1186/s13018-024-04920-8. J Orthop Surg Res. 2024. PMID: 39061045 Free PMC article.
-
The Effect of Body Position and the Reliability of Upper Limb Arterial Occlusion Pressure Using a Handheld Doppler Ultrasound for Blood Flow Restriction Training.Sports Health. 2022 Sep-Oct;14(5):717-724. doi: 10.1177/19417381211043877. Epub 2021 Sep 13. Sports Health. 2022. PMID: 34515589 Free PMC article. Clinical Trial.
-
Validity and reliability of a wearable blood flow restriction training device for arterial occlusion pressure assessment.Front Physiol. 2024 Jun 7;15:1404247. doi: 10.3389/fphys.2024.1404247. eCollection 2024. Front Physiol. 2024. PMID: 38911327 Free PMC article.
-
Efficacy and acceptability of different blood flow restriction training interventions during the rehabilitation of military personnel with lower limb musculoskeletal injuries: protocol for a two-phase randomised controlled trial.BMJ Open. 2025 May 26;15(5):e096643. doi: 10.1136/bmjopen-2024-096643. BMJ Open. 2025. PMID: 40425246 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical