Early onset senescence and cognitive impairment in a murine model of repeated mTBI
- PMID: 33964983
- PMCID: PMC8106230
- DOI: 10.1186/s40478-021-01190-x
Early onset senescence and cognitive impairment in a murine model of repeated mTBI
Abstract
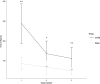
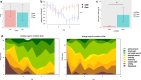
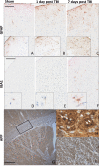
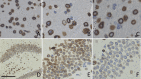
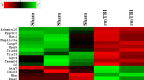
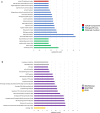
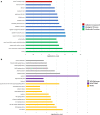
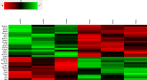
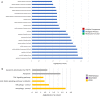
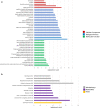
Mild traumatic brain injury (mTBI) results in broad neurological symptoms and an increased risk of being diagnosed with a neurodegenerative disease later in life. While the immediate oxidative stress response and post-mortem pathology of the injured brain has been well studied, it remains unclear how early pathogenic changes may drive persistent symptoms and confer susceptibility to neurodegeneration. In this study we have used a mouse model of repeated mTBI (rmTBI) to identify early gene expression changes at 24 h or 7 days post-injury (7 dpi). At 24 h post-injury, gene expression of rmTBI mice shows activation of the DNA damage response (DDR) towards double strand DNA breaks, altered calcium and cell-cell signalling, and inhibition of cell death pathways. By 7 dpi, rmTBI mice had a gene expression signature consistent with induction of cellular senescence, activation of neurodegenerative processes, and inhibition of the DDR. At both timepoints gliosis, microgliosis, and axonal damage were evident in the absence of any gross lesion, and by 7 dpi rmTBI also mice had elevated levels of IL1β, p21, 53BP1, DNA2, and p53, supportive of DNA damage-induced cellular senescence. These gene expression changes reflect establishment of processes usually linked to brain aging and suggests that cellular senescence occurs early and most likely prior to the accumulation of toxic proteins. These molecular changes were accompanied by spatial learning and memory deficits in the Morris water maze. To conclude, we have identified DNA damage-induced cellular senescence as a repercussion of repeated mild traumatic brain injury which correlates with cognitive impairment. Pathways involved in senescence may represent viable treatment targets of post-concussive syndrome. Senescence has been proposed to promote neurodegeneration and appears as an effective target to prevent long-term complications of mTBI, such as chronic traumatic encephalopathy and other related neurodegenerative pathologies.
Keywords: Ageing; Concussion; DNA damage response; Neurodegeneration; Neuroinflammation; Senescence; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

