Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s
- PMID: 33965091
- PMCID: PMC8114584
- DOI: 10.1016/j.jaci.2021.03.015
Roles of innate lymphoid cells (ILCs) in allergic diseases: The 10-year anniversary for ILC2s
Abstract
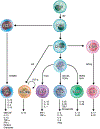
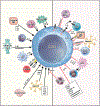
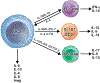
In the 12 years since the discovery of innate lymphoid cells (ILCs), our knowledge of their immunobiology has expanded rapidly. Group 2 ILCs (ILC2s) respond rapidly to allergen exposure and environmental insults in mucosal organs, producing type 2 cytokines. Early studies showed that epithelium-derived cytokines activate ILC2s, resulting in eosinophilia, mucus hypersecretion, and remodeling of mucosal tissues. We now know that ILC2s are regulated by other cytokines, eicosanoids, and neuropeptides as well, and interact with both immune and stromal cells. Furthermore, ILC2s exhibit plasticity by adjusting their functions depending on their tissue environment and may consist of several heterogeneous subpopulations. Clinical studies show that ILC2s are involved in asthma, allergic rhinitis, chronic rhinosinusitis, food allergy, and eosinophilic esophagitis. However, much remains unknown about the immunologic mechanisms involved. Beneficial functions of ILCs in maintenance or restoration of tissue well-being and human health also need to be clarified. As our understanding of the crucial functions ILCs play in both homeostasis and disease pathology expands, we are poised to make tremendous strides in diagnostic and therapeutic options for patients with allergic diseases. This review summarizes discoveries in immunobiology of ILCs and their roles in allergic diseases in the past 5 years, discusses controversies and gaps in our knowledge, and suggests future research directions.
Keywords: Innate lymphoid cells; allergic rhinitis; asthma; atopic dermatitis; chronic rhinosinusitis; eosinophilic esophagitis; group 2 innate lymphoid cells.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Boonpiyathad T, Sozener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333. - PubMed
-
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15:985–95. - PubMed
-
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

