Activating mGlu3 Metabotropic Glutamate Receptors Rescues Schizophrenia-like Cognitive Deficits Through Metaplastic Adaptations Within the Hippocampus
- PMID: 33965197
- PMCID: PMC8403106
- DOI: 10.1016/j.biopsych.2021.02.970
Activating mGlu3 Metabotropic Glutamate Receptors Rescues Schizophrenia-like Cognitive Deficits Through Metaplastic Adaptations Within the Hippocampus
Abstract
Background: Polymorphisms in GRM3, the gene encoding the mGlu3 metabotropic glutamate receptor, are associated with impaired cognition and neuropsychiatric disorders such as schizophrenia. Limited availability of selective genetic and molecular tools has hindered progress in developing a clear understanding of the mechanisms through which mGlu3 receptors regulate synaptic plasticity and cognition.
Methods: We examined associative learning in mice with trace fear conditioning, a hippocampal-dependent learning task disrupted in patients with schizophrenia. Underlying cellular mechanisms were assessed using ex vivo hippocampal slice preparations with selective pharmacological tools and selective genetic deletion of mGlu3 receptor expression in specific neuronal subpopulations.
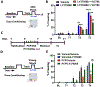
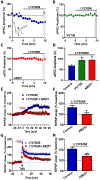
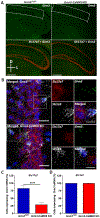
Results: mGlu3 receptor activation enhanced trace fear conditioning and reversed deficits induced by subchronic phencyclidine. Mechanistic studies revealed that mGlu3 receptor activation induced metaplastic changes, biasing afferent stimulation to induce long-term potentiation through an mGlu5 receptor-dependent, endocannabinoid-mediated, disinhibitory mechanism. Selective genetic deletion of either mGlu3 or mGlu5 from hippocampal pyramidal cells eliminated effects of mGlu3 activation, revealing a novel mechanism by which mGlu3 and mGlu5 interact to enhance cognitive function.
Conclusions: These data demonstrate that activation of mGlu3 receptors in hippocampal pyramidal cells enhances hippocampal-dependent cognition in control and impaired mice by inducing a novel form of metaplasticity to regulate circuit function, providing a clear mechanism through which genetic variation in GRM3 can contribute to cognitive deficits. Developing approaches to positively modulate mGlu3 receptor function represents an encouraging new avenue for treating cognitive disruption in schizophrenia and other psychiatric diseases.
Keywords: Cognition; Hippocampus; Schizophrenia; Synaptic plasticity; mGlu(3); mGlu(5).
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
P.J.C., C.W.L, and C.M.N. receive research support from Acadia Pharmaceuticals and Boehringer Ingelheim and C.W.L. also receives support from Ono Pharmaceutical. P.J.C., C.W.L., and C.M.N. are inventors on multiple patents for allosteric modulators of metabotropic glutamate receptors. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures





Comment in
-
mGlu3 Metabotropic Glutamate Receptors-New Hope for Pharmacotherapy of Schizophrenia.Biol Psychiatry. 2021 Sep 15;90(6):356-358. doi: 10.1016/j.biopsych.2021.06.014. Biol Psychiatry. 2021. PMID: 34446153 Free PMC article. No abstract available.
References
-
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA (2008): The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 22:308–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 MH111124/MH/NIMH NIH HHS/United States
- R01 NS112171/NS/NINDS NIH HHS/United States
- R01 NS031373/NS/NINDS NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P50 HD103537/HD/NICHD NIH HHS/United States
- R37 NS031373/NS/NINDS NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- K99 AA027806/AA/NIAAA NIH HHS/United States
- R01 MH062646/MH/NIMH NIH HHS/United States
- K01 MH112983/MH/NIMH NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

