HPV vaccination to prevent recurrence of anal intraepithelial neoplasia in HIV+ MSM
- PMID: 33966029
- PMCID: PMC8373452
- DOI: 10.1097/QAD.0000000000002928
HPV vaccination to prevent recurrence of anal intraepithelial neoplasia in HIV+ MSM
Abstract
Objective: Anal cancer precursor lesions high-grade anal intraepithelial neoplasia (HGAIN) are highly prevalent among HIV+ MSM. Treatment of HGAIN is frustrated by high recurrence rates. We investigated the efficacy of the quadrivalent human papillomavirus (qHPV) vaccine as posttreatment adjuvant in preventing HGAIN recurrence in HIV+ MSM.
Design: Randomized, double-blind, placebo-controlled, multicentre trial.
Setting: Three HIV outpatient clinics in Amsterdam, the Netherlands.
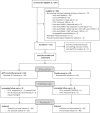
Subjects: HIV+ MSM with CD4+ cell count more than 350 cells/μl, biopsy-proven intra-anal HGAIN successfully treated in the past year, and lesions still in remission at enrolment, as assessed by high-resolution anoscopy (HRA).
Intervention: Participants were randomized to three doses of qHPV (Gardasil-4, MSD) or placebo with vaccinations at 0, 2, and 6 months. HRA was repeated at 6, 12, and 18 months.
Main outcome measure: The primary outcome was cumulative, biopsy-proven HGAIN recurrence rate at 18 months, evaluated in an intention-to-treat (ITT) (received all vaccinations) and per-protocol analysis (all vaccinations and complete follow-up).
Results: We randomized 126 participants of which 64 (50.8%) received qHPV and 62 (49.2%) placebo. All participants received three vaccinations, and in both groups for two participants follow-up was incomplete. We found no difference (P = 0.38) in cumulative HGAIN recurrence rates between the qHPV (44/64, 68.8%) and placebo group (38/62, 61.3%) in the ITT analysis [absolute risk reduction -7.5 (95% confidence interval (CI) -24.1 to 9.2)]. This was similar in the per-protocol analysis.
Conclusion: Despite adequate serological responses to qHPV vaccination, short-term recurrence of HGAIN was not prevented. These findings do not support qHPV vaccination as a treatment adjuvant to prevent HGAIN recurrence in HIV+ MSM.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.G.W.D. has a family member working at MSD. H.J.C.d.V. received money or goods for research from Medigene, Gilead and MSD, money for presentations from Abbott and Janssen and money for advice from Medigene and Novartis. All other authors have no conflicts of interest to declare.
Figures
Comment in
-
Anal neoplasia: prevention or treatment?AIDS. 2021 Sep 1;35(11):1863-1865. doi: 10.1097/QAD.0000000000002969. AIDS. 2021. PMID: 34397485 No abstract available.
References
-
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383. - PubMed
-
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010; 10:550–560. - PubMed
-
- Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous