SARS-CoV-2 vaccines: Lights and shadows
- PMID: 33966930
- PMCID: PMC8084611
- DOI: 10.1016/j.ejim.2021.04.019
SARS-CoV-2 vaccines: Lights and shadows
Abstract
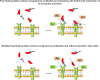
Vaccines to prevent acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection elicit an immune neutralizing response. Some concerns have been raised regarding the safety of SARS-CoV-2 vaccines, largely based on case-reports of serious thromboembolic events after vaccination. Some mechanisms have been suggested which might explain the adverse cardiovascular reactions to SARS-CoV-2 vaccines. Different vaccine platforms are currently available which include live attenuated vaccines, inactivated vaccines, recombinant protein vaccines, vector vaccines, DNA vaccines and RNA vaccines. Vaccines increase the endogenous synthesis of SARS-CoV-2 Spike proteins from a variety of cells. Once synthetized, the Spike proteins assembled in the cytoplasma migrate to the cell surface and protrude with a native-like conformation. These proteins are recognized by the immune system which rapidly develops an immune response. Such response appears to be quite vigorous in the presence of DNA vaccines which encode viral vectors, as well as in subjects who are immunized because of previous exposure to SARS-CoV-2. The resulting pathological features may resemble those of active coronavirus disease. The free-floating Spike proteins synthetized by cells targeted by vaccine and destroyed by the immune response circulate in the blood and systematically interact with angiotensin converting enzyme 2 (ACE2) receptors expressed by a variety of cells including platelets, thereby promoting ACE2 internalization and degradation. These reactions may ultimately lead to platelet aggregation, thrombosis and inflammation mediated by several mechanisms including platelet ACE2 receptors. Whereas Phase III vaccine trials generally excluded participants with previous immunization, vaccination of huge populations in the real life will inevitably include individuals with preexisting immunity. This might lead to excessively enhanced inflammatory and thrombotic reactions in occasional subjects. Further research is urgently needed in this area.
Keywords: ACE2; Adverse event; COVID-19; Renin-angiotensin-aldosterone system; SARS-CoV-2; Thrombosis; Vaccines.
Copyright © 2021 European Federation of Internal Medicine. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors of this study has financial or other reasons that could lead to a conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

