Longitudinal Cerebral Blood Flow Changes in Normal Aging and the Alzheimer's Disease Continuum Identified by Arterial Spin Labeling MRI
- PMID: 33967053
- PMCID: PMC8217256
- DOI: 10.3233/JAD-210116
Longitudinal Cerebral Blood Flow Changes in Normal Aging and the Alzheimer's Disease Continuum Identified by Arterial Spin Labeling MRI
Abstract
Background: Cross-sectional studies have shown lower cerebral blood flow (CBF) in Alzheimer's disease (AD), but longitudinal CBF changes in AD are still unknown.
Objective: To reveal the longitudinal CBF changes in normal control (NC) and the AD continuum using arterial spin labeling perfusion magnetic resonance imaging (ASL MRI).
Methods: CBF was calculated from two longitudinal ASL scans acquired 2.22±1.43 years apart from 140 subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI). At the baseline scan, the cohort contained 41 NC, 74 mild cognitive impairment patients (MCI), and 25 AD patients. 21 NC converted into MCI and 17 MCI converted into AD at the follow-up. Longitudinal CBF changes were assessed using paired-t test for non-converters and converters separately at each voxel and in the meta-ROI. Age and sex were used as covariates.
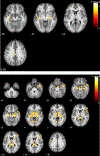
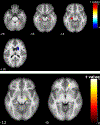
Results: CBF reductions were observed in all subjects. Stable NC (n = 20) showed CBF reduction in the hippocampus and precuneus. Stable MCI patients (n = 57) showed spatially more extended CBF reduction patterns in hippocampus, middle temporal lobe, ventral striatum, prefrontal cortex, and cerebellum. NC-MCI converters showed CBF reduction in hippocampus and cerebellum and CBF increase in caudate. MCI-AD converters showed CBF reduction in hippocampus and prefrontal cortex. CBF changes were not related with longitudinal neurocognitive changes.
Conclusion: Normal aging and AD continuum showed common longitudinal CBF reductions in hippocampus independent of disease and its conversion. Disease conversion independent longitudinal CBF reductions escalated in MCI subjects.
Keywords: Aging; Alzheimer’s disease; arterial spin labeling; cerebral blood flow; longitudinal analysis.
Conflict of interest statement
CONFLICT OF INTEREST/DISCLOSURE STATEMENT
The authors have no conflict of interest to report.
Figures
References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. - PMC - PubMed
-
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R; Contributors (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. - PMC - PubMed
-
- Alsop DC, Detre JA, Grossman M (2000) Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 47, 93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

