Cerebrotendinous Xanthomatosis: Molecular Pathogenesis, Clinical Spectrum, Diagnosis, and Disease-Modifying Treatments
- PMID: 33967188
- PMCID: PMC8532057
- DOI: 10.5551/jat.RV17055
Cerebrotendinous Xanthomatosis: Molecular Pathogenesis, Clinical Spectrum, Diagnosis, and Disease-Modifying Treatments
Abstract
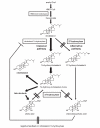
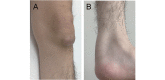
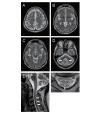
Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive lipid storage disorder caused by mutations in the CYP27A1 gene, which encodes the mitochondrial enzyme sterol 27-hydroxylase. Decreased sterol 27-hydroxylase activity results in impaired bile acid synthesis, leading to reduced production of bile acids, especially chenodeoxycholic acid (CDCA), as well as elevated serum cholestanol and urine bile alcohols. The accumulation of cholestanol and cholesterol mainly in the brain, lenses, and tendons results in the characteristic clinical manifestations of CTX. Clinical presentation is characterized by systemic symptoms including neonatal jaundice or cholestasis, refractory diarrhea, juvenile cataracts, tendon xanthomas, osteoporosis, coronary heart disease, and a broad range of neuropsychiatric manifestations. The combinations of symptoms vary from patient to patient and the presenting symptoms, especially in the early disease phase, may be nonspecific, which leads to a substantial diagnostic delay or underdiagnosis. Replacement of CDCA has been approved as a first-line treatment for CTX, and can lead to biochemical and clinical improvements. However, the effect of CDCA treatment is limited once significant neuropsychiatric manifestations are established. The age at diagnosis and initiation of CDCA treatment correlate with the prognosis of patients with CTX. Therefore, early diagnosis and subsequent treatment initiation are essential.
Keywords: CTX; CYP27A1; Cerebrotendinous xanthomatosis; Chenodeoxycholic acid; Cholestanol.
Figures

References
-
- Verrips A, Hoefsloot LH, Steenbergen GC, Theelen JP, Wevers RA, Gabreëls FJ, van Engelen BG, van den Heuvel LP: Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain, 2000; 123: 908-919 - PubMed
-
- Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR, Pardo J, Arias M, Ares-Luque A, Duarte J, Muñiz-Pérez S, Sobrido MJ: Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol, 2011; 18: 1203-1211 - PubMed
-
- Mignarri A, Gallus GN, Dotti MT, Federico A: A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis, 2014; 37: 421-429 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

