Depletion of MRPL35 inhibits gastric carcinoma cell proliferation by regulating downstream signaling proteins
- PMID: 33967557
- PMCID: PMC8072187
- DOI: 10.3748/wjg.v27.i16.1785
Depletion of MRPL35 inhibits gastric carcinoma cell proliferation by regulating downstream signaling proteins
Abstract
Background: Gastric carcinoma (GC) is a digestive system disease with high morbidity and mortality. However, early clinical detection is difficult, and the therapeutic effect for advanced disease is not satisfactory. Thus, finding new tumor markers and therapeutic targets conducive to the treatment of GC is imperative. MRPL35 is a member of the large subunit family of mitochondrial ribosomal protein. MRPL35 shows the characteristic of oncogene in colorectal cancer and esophageal cancer, which promotes the exploration of the correlation between MRPL35 and GC. We proposed that the expression of MRPL35 might be critical in GC.
Aim: To study the effect of MRPL35 knockdown on GC cell proliferation.
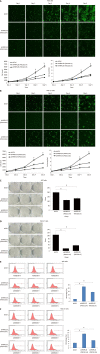
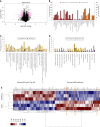
Methods: The expression of MRPL35 in GC was evaluated based on data from the public tumor database UALCAN (www.ualcan.path.uab.edu). The effect of the expression of MRPL35 on the prognosis was evaluated with KMplot (www.kmplot.com). The expression of MRPL35 was assessed on the tissue microarray by immunohistochemistry and the level of MRPL35 mRNA in 25 pairs of clinical GC tissues and matched adjacent tissues was detected by quantitative reverse transcription-polymerase chain reaction. Celigo cell count assay, colony formation assay, and flow cytometry were used to assess the role of MRPL35 in GC cell proliferation and apoptosis in vitro. Additionally, tumor formation experiment in BALB/c nude mice was utilized to determine the effect of MRPL35 on GC cell proliferation. After knockdown of MRPL35, related proteins were identified by isobaric tags for relative and absolute quantification analysis, and the expression of related proteins was detected by Western blot.
Results: The expression of MRPL35 was up-regulated in GC (P = 1.77 × 10-4). The Kaplan-Meier plots of the overall survival indicated that high expression of MRPL35 was associated with a poor survival in GC. Compared with adjacent tissues, the expression of MRPL35 in GC tissues was increased, which was related to age (P = 0.03), lymph node metastasis (P = 0.007), and pathological tumor-node-metastasis stage (P = 0.024). Knockdown of MRPL35 inhibited GC cell proliferation and colony formation and induced apoptosis. Animal experiment results showed that knockdown of MRPL35 inhibited tumor formation in BALB/c nude mice. Western blotting analysis showed that after knockdown of MRPL35, the expression of PICK1 and BCL-XL proteins decreased, and that of AGR2 protein increased.
Conclusion: Collectively, our findings demonstrate that knockdown of MRPL35 inhibits GC cell proliferation through related proteins including PICK1, BCL-XL, and AGR2.
Keywords: Apoptosis; Gastric carcinoma; Isobaric tags for relative and absolute quantification; MRPL35; Proliferation; Tissue microarray.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no financial or commercial conflict of interest.
Figures





Similar articles
-
Knockdown of MRPL35 promotes cell apoptosis and inhibits cell proliferation in non-small-cell lung cancer.BMC Pulm Med. 2023 Dec 13;23(1):507. doi: 10.1186/s12890-023-02677-0. BMC Pulm Med. 2023. PMID: 38093266 Free PMC article.
-
MRPL35 Induces Proliferation, Invasion, and Glutamine Metabolism in NSCLC Cells by Upregulating SLC7A5 Expression.Clin Respir J. 2024 Jul;18(7):e13799. doi: 10.1111/crj.13799. Clin Respir J. 2024. PMID: 38987867 Free PMC article.
-
18β-glycyrrhetinic acid regulates mitochondrial ribosomal protein L35-associated apoptosis signaling pathways to inhibit proliferation of gastric carcinoma cells.World J Gastroenterol. 2022 Jun 14;28(22):2437-2456. doi: 10.3748/wjg.v28.i22.2437. World J Gastroenterol. 2022. PMID: 35979263 Free PMC article.
-
MRPL35 Is Up-Regulated in Colorectal Cancer and Regulates Colorectal Cancer Cell Growth and Apoptosis.Am J Pathol. 2019 May;189(5):1105-1120. doi: 10.1016/j.ajpath.2019.02.003. Epub 2019 Mar 9. Am J Pathol. 2019. PMID: 30862482
-
One stomach, two subtypes of carcinoma-the differences between distal and proximal gastric cancer.Gastroenterol Rep (Oxf). 2021 Nov 15;9(6):489-504. doi: 10.1093/gastro/goab050. eCollection 2021 Dec. Gastroenterol Rep (Oxf). 2021. PMID: 34925847 Free PMC article. Review.
Cited by
-
Knockdown of MRPL35 promotes cell apoptosis and inhibits cell proliferation in non-small-cell lung cancer.BMC Pulm Med. 2023 Dec 13;23(1):507. doi: 10.1186/s12890-023-02677-0. BMC Pulm Med. 2023. PMID: 38093266 Free PMC article.
-
Mitochondrial Ribosomal Proteins and Cancer.Medicina (Kaunas). 2025 Jan 9;61(1):96. doi: 10.3390/medicina61010096. Medicina (Kaunas). 2025. PMID: 39859078 Free PMC article. Review.
-
Identification and validation of a fatty acid metabolism gene signature for the promotion of metastasis in liver cancer.Oncol Lett. 2023 Sep 6;26(4):457. doi: 10.3892/ol.2023.14044. eCollection 2023 Oct. Oncol Lett. 2023. PMID: 37736554 Free PMC article.
-
MRPL35 Induces Proliferation, Invasion, and Glutamine Metabolism in NSCLC Cells by Upregulating SLC7A5 Expression.Clin Respir J. 2024 Jul;18(7):e13799. doi: 10.1111/crj.13799. Clin Respir J. 2024. PMID: 38987867 Free PMC article.
-
18β-glycyrrhetinic acid regulates mitochondrial ribosomal protein L35-associated apoptosis signaling pathways to inhibit proliferation of gastric carcinoma cells.World J Gastroenterol. 2022 Jun 14;28(22):2437-2456. doi: 10.3748/wjg.v28.i22.2437. World J Gastroenterol. 2022. PMID: 35979263 Free PMC article.
References
-
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

