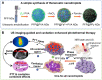
A Highly Efficient One-for-All Nanodroplet for Ultrasound Imaging-Guided and Cavitation-Enhanced Photothermal Therapy
- PMID: 33967577
- PMCID: PMC8096805
- DOI: 10.2147/IJN.S301734
A Highly Efficient One-for-All Nanodroplet for Ultrasound Imaging-Guided and Cavitation-Enhanced Photothermal Therapy
Abstract
Background: Photothermal therapy (PTT) has attracted considerable attention for cancer treatment as it is highly controllable and minimally invasive. Various multifunctional nanosystems have been fabricated in an "all-in-one" form to guide and enhance PTT by integrating imaging and therapeutic functions. However, the complex fabrication of nanosystems and their high cost limit its clinical translation.
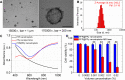
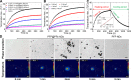
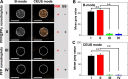
Materials and methods: Herein, a high efficient "one-for-all" nanodroplet with a simple composition but owning multiple capabilities was developed to achieve ultrasound (US) imaging-guided and cavitation-enhanced PTT. Perfluoropentane (PFP) nanodroplet with a polypyrrole (PPy) shell (PFP@PPy nanodroplet) was synthesized via ultrasonic emulsification and in situ oxidative polymerization. After characterization of the morphology, its photothermal effect, phase transition performance, as well as its capabilities of enhancing US imaging and acoustic cavitation were examined. Moreover, the antitumor efficacy of the combined therapy with PTT and acoustic cavitation via the PFP@PPy nanodroplets was studied both in vitro and in vivo.
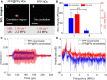
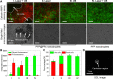
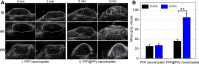
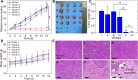
Results: The nanodroplets exhibited good stability, high biocompatibility, broad optical absorption over the visible and near-infrared (NIR) range, excellent photothermal conversion with an efficiency of 60.1% and activatable liquid-gas phase transition performance. Upon NIR laser and US irradiation, the phase transition of PFP cores into microbubbles significantly enhanced US imaging and acoustic cavitation both in vitro and in vivo. More importantly, the acoustic cavitation enhanced significantly the antitumor efficacy of PTT as compared to PTT alone thanks to the cavitation-mediated cell destruction, which demonstrated a substantial increase in cell detachment, 81.1% cell death in vitro and 99.5% tumor inhibition in vivo.
Conclusion: The PFP@PPy nanodroplet as a "one-for-all" theranostic agent achieved highly efficient US imaging-guided and cavitation-enhanced cancer therapy, and has considerable potential to provide cancer theranostics in the future.
Keywords: acoustic cavitation; one-for-all nanodroplet; photothermal therapy; theranostics; ultrasound imaging.
© 2021 Qin et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
A mesoporous theranostic platform for ultrasound and photoacoustic dual imaging-guided photothermal and enhanced starvation therapy for cancer.Acta Biomater. 2024 Jul 15;183:264-277. doi: 10.1016/j.actbio.2024.05.040. Epub 2024 May 28. Acta Biomater. 2024. PMID: 38815685
-
Polypyrrole-coated phase-change liquid perfluorocarbon nanoparticles for the visualized photothermal-chemotherapy of breast cancer.Acta Biomater. 2019 May;90:337-349. doi: 10.1016/j.actbio.2019.03.056. Epub 2019 Mar 29. Acta Biomater. 2019. PMID: 30936037
-
Gold Nanorod-Loaded Nano-Contrast Agent with Composite Shell-Core Structure for Ultrasonic/Photothermal Imaging-Guided Therapy in Ischemic Muscle Disorders.Int J Nanomedicine. 2024 May 8;19:4121-4136. doi: 10.2147/IJN.S445990. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38736655 Free PMC article.
-
Functional ultrasound-triggered phase-shift perfluorocarbon nanodroplets for cancer therapy.Ultrasound Med Biol. 2021 Aug;47(8):2064-2079. doi: 10.1016/j.ultrasmedbio.2021.04.003. Epub 2021 May 13. Ultrasound Med Biol. 2021. PMID: 33992473 Review.
-
Carbon-based Nanomaterials in Photothermal Therapy Guided by Photoacoustic Imaging: State of Knowledge and Recent Advances.Curr Med Chem. 2025;32(2):238-257. doi: 10.2174/0109298673287448240311112523. Curr Med Chem. 2025. PMID: 38529603 Review.
Cited by
-
Nanomaterials for Ultrasound Imaging- Guided Sonodynamic Therapy.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241263197. doi: 10.1177/15330338241263197. Technol Cancer Res Treat. 2024. PMID: 39051705 Free PMC article. Review.
-
The hybrid nanosystem for the identification and magnetic hyperthermia immunotherapy of metastatic sentinel lymph nodes as a multifunctional theranostic agent.Front Bioeng Biotechnol. 2024 Jul 29;12:1445829. doi: 10.3389/fbioe.2024.1445829. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39135950 Free PMC article.
-
Phase-transition nanodroplets with immunomodulatory capabilities for potentiating mild magnetic hyperthermia to inhibit tumour proliferation and metastasis.J Nanobiotechnology. 2023 Apr 17;21(1):131. doi: 10.1186/s12951-023-01885-4. J Nanobiotechnology. 2023. PMID: 37069614 Free PMC article.
-
Photoacoustic Properties of Polypyrrole Nanoparticles.Nanomaterials (Basel). 2021 Sep 21;11(9):2457. doi: 10.3390/nano11092457. Nanomaterials (Basel). 2021. PMID: 34578773 Free PMC article.
-
Synergy of dissolving microneedles and ultrasound to enhance transdermal delivery for rheumatoid arthritis.Drug Deliv Transl Res. 2025 May 15. doi: 10.1007/s13346-025-01876-y. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40372697
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

