Mitochondrial CHCHD2: Disease-Associated Mutations, Physiological Functions, and Current Animal Models
- PMID: 33967741
- PMCID: PMC8100248
- DOI: 10.3389/fnagi.2021.660843
Mitochondrial CHCHD2: Disease-Associated Mutations, Physiological Functions, and Current Animal Models
Abstract
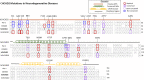
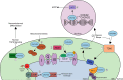
Rare mutations in the mitochondrial protein coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2) are associated with Parkinson's disease (PD) and other Lewy body disorders. CHCHD2 is a bi-organellar mediator of oxidative phosphorylation, playing crucial roles in regulating electron flow in the mitochondrial electron transport chain and acting as a nuclear transcription factor for a cytochrome c oxidase subunit (COX4I2) and itself in response to hypoxic stress. CHCHD2 also regulates cell migration and differentiation, mitochondrial cristae structure, and apoptosis. In this review, we summarize the known disease-associated mutations of CHCHD2 in Asian and Caucasian populations, the physiological functions of CHCHD2, how CHCHD2 mutations contribute to α-synuclein pathology, and current animal models of CHCHD2. Further, we discuss the necessity of continued investigation into the divergent functions of CHCHD2 and CHCHD10 to determine how mutations in these similar mitochondrial proteins contribute to different neurodegenerative diseases.
Keywords: CHCHD10; CHCHD2; Lewy body disorders; Parkinson’s disease; mitochondria.
Copyright © 2021 Kee, Espinoza Gonzalez, Wehinger, Bukhari, Ermekbaeva, Sista, Kotsiviras, Liu, Kang and Woo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

