Yi-Qi-Jian-Pi Formula Suppresses RIPK1/RIPK3-Complex-Dependent Necroptosis of Hepatocytes Through ROS Signaling and Attenuates Liver Injury in Vivo and in Vitro
- PMID: 33967802
- PMCID: PMC8102982
- DOI: 10.3389/fphar.2021.658811
Yi-Qi-Jian-Pi Formula Suppresses RIPK1/RIPK3-Complex-Dependent Necroptosis of Hepatocytes Through ROS Signaling and Attenuates Liver Injury in Vivo and in Vitro
Abstract
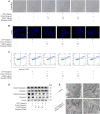
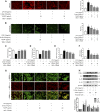
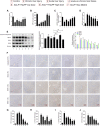
Acute-on-chronic liver failure (ACLF) is described as a characteristic of acute jaundice and coagulation dysfunction. Effective treatments for ACLF are unavailable and hence are urgently required. We aimed to define the effect of Yi-Qi-Jian-Pi Formula (YQJPF) on liver injury and further examine the molecular mechanisms. In this study, we established CCl4-, LPS-, and d-galactosamine (D-Gal)-induced ACLF rat models in vivo and LPS- and D-Gal-induced hepatocyte injury models in vitro. We found that YQJPF significantly ameliorates liver injury in vivo and in vitro that is associated with the regulation of hepatocyte necroptosis. Specifically, YQJPF decreased expression of receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3) and pseudokinase mixed lineage kinase domain-like (MLKL) to inhibit the migration of RIPK1 and RIPK3 into necrosome. YQJPF also reduces the expression of inflammatory cytokines IL-6, IL-8, IL-1β, and TNF-α, which were regulated by RIPK3 mediates cell death. RIPK1 depletion was found to enhance the protective effect of YQJPF. Furthermore, we showed that YQJPF significantly downregulates the mitochondrial reactive oxygen species (ROS) production and mitochondrial depolarization, with ROS scavenger, 4-hydroxy-TEMPO treatment recovering impaired RIPK1-mediated necroptosis and reducing the expression of IL-6, IL-8, IL-1β, and TNF-α. In summary, our study revealed the molecular mechanism of protective effect of YQJPF on hepatocyte necroptosis, targeting RIPK1/RIPK3-complex-dependent necroptosis via ROS signaling. Overall, our results provided a novel perspective to indicate the positive role of YQJPF in ACLF.
Keywords: Yi-Qi-Jian-Pi formula; acute-on-chronic liver failure; hepatocytes necroptosis; inflammation; reactive oxygen species.
Copyright © 2021 Wang, Tang, Liang, Jin, Gao, Li, Li, Shao, Zhang, Tan, Zhang and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Yi-Qi-Jian-Pi formula inhibits hepatocyte pyroptosis through the IDH2-driven tricarboxylic acid cycle to reduce liver injury in acute-on-chronic liver failure.J Ethnopharmacol. 2023 Dec 5;317:116683. doi: 10.1016/j.jep.2023.116683. Epub 2023 Jun 13. J Ethnopharmacol. 2023. PMID: 37315653
-
Yi-Qi-Jian-Pi formula modulates the PI3K/AKT signaling pathway to attenuate acute-on-chronic liver failure by suppressing hypoxic injury and apoptosis in vivo and in vitro.J Ethnopharmacol. 2021 Nov 15;280:114411. doi: 10.1016/j.jep.2021.114411. Epub 2021 Jul 12. J Ethnopharmacol. 2021. PMID: 34265380
-
Yi-Qi-Jian-Pi formula ameliorates immune function in acute-on-chronic liver failure by upregulating autophagy and mitochondrial biogenesis in CD8+ T lymphocytes.J Ethnopharmacol. 2023 May 23;308:116276. doi: 10.1016/j.jep.2023.116276. Epub 2023 Feb 17. J Ethnopharmacol. 2023. PMID: 36806340
-
Mitochondrial Mechanisms of Necroptosis in Liver Diseases.Int J Mol Sci. 2020 Dec 23;22(1):66. doi: 10.3390/ijms22010066. Int J Mol Sci. 2020. PMID: 33374660 Free PMC article. Review.
-
The Receptor Interacting Protein Kinases in the Liver.Semin Liver Dis. 2018 Feb;38(1):73-86. doi: 10.1055/s-0038-1629924. Epub 2018 Feb 22. Semin Liver Dis. 2018. PMID: 29471568 Free PMC article. Review.
Cited by
-
Prospect of Animal Models for Acute-on-chronic Liver Failure: A Mini-review.J Clin Transl Hepatol. 2022 Oct 28;10(5):995-1003. doi: 10.14218/JCTH.2022.00086. Epub 2022 Apr 28. J Clin Transl Hepatol. 2022. PMID: 36304511 Free PMC article. Review.
-
Roles of necroptosis in alcoholic liver disease and hepatic pathogenesis.Cell Prolif. 2022 Mar;55(3):e13193. doi: 10.1111/cpr.13193. Epub 2022 Jan 26. Cell Prolif. 2022. PMID: 35083817 Free PMC article. Review.
-
Chinese herbal decoction, Yi-Qi-Jian-Pi formula exerts anti-hepatic fibrosis effects in mouse models of CCl4-induced liver fibrosis.Heliyon. 2024 Feb 22;10(5):e26129. doi: 10.1016/j.heliyon.2024.e26129. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434258 Free PMC article.
-
Cpd-42 protects against calcium oxalate nephrocalcinosis-induced renal injury and inflammation by targeting RIPK3-mediated necroptosis.Front Pharmacol. 2022 Nov 3;13:1041117. doi: 10.3389/fphar.2022.1041117. eCollection 2022. Front Pharmacol. 2022. PMID: 36408256 Free PMC article.
-
Ancient Herbal Formula Mahuang Lianqiao Chixiaodou Decoction Protects Acute and Acute-on-Chronic Liver Failure via Inhibiting von Willebrand Factor Signaling.Cells. 2022 Oct 25;11(21):3368. doi: 10.3390/cells11213368. Cells. 2022. PMID: 36359765 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

