The Efficacy and Safety of Pulmonary Vasodilators in Pediatric Pulmonary Hypertension (PH): A Systematic Review and Meta-analysis
- PMID: 33967811
- PMCID: PMC8103162
- DOI: 10.3389/fphar.2021.668902
The Efficacy and Safety of Pulmonary Vasodilators in Pediatric Pulmonary Hypertension (PH): A Systematic Review and Meta-analysis
Abstract
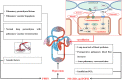
Background: We performed a meta-analysis to evaluate the efficacy and safety of pulmonary vasodilators in pediatric pulmonary hypertension (PH) patients. Methods: We searched electronic databases including PubMed, EMBASE, and the Cochrane Library up to May 2020, and conducted a subgroup analysis for pulmonary vasodilators or underlying disease. Results: Fifteen studies with 719 pediatric PH patients were included in the meta-analysis. Adverse events did not differ (p = 0.11, I 2 = 15%) between the pulmonary vasodilators group and the control group, neither in the subgroups. In total, compared with the control group treatment, pulmonary vasodilators significantly decreased the mortality (p = 0.002), mean pulmonary artery pressure (mPAP, p = 0.02), and mechanical ventilation duration (p = 0.03), also improved the oxygenation index (OI, p = 0.01). In the persistent pulmonary hypertension of the newborn (PPHN) subgroup, phosphodiesterase type 5 inhibitors (PDE5i) significantly reduced mortality (p = 0.03), OI (p = 0.007) and mechanical ventilation duration (p = 0.004). Administration of endothelin receptor antagonists (ERAs) improved OI (p = 0.04) and mechanical ventilation duration (p < 0.00001) in PPHN. We also found that in the pediatric pulmonary arterial hypertension (PPAH) subgroup, mPAP was pronouncedly declined with ERAs (p = 0.006). Systolic pulmonary artery pressure (sPAP, p < 0.0001) and pulmonary arterial/aortic pressure (PA/AO, p < 0.00001) were significantly relieved with PDE5i, partial pressure of arterial oxygen (PaO2) was improved with prostacyclin in postoperative PH (POPH) subgroup (p = 0.001). Compared with the control group, pulmonary vasodilators could significantly decrease PA/AO pressure (p < 0.00001) and OI (p < 0.00001) in the short-term (duration <7 days) follow-up subgroup, improve mPAP (p = 0.03) and PaO2 (p = 0.01) in the mid-term (7-30 days) follow-up subgroup, also decrease mortality, mPAP (p = 0.0001), PA/AO pressure (p = 0.0007), duration of mechanical ventilation (p = 0.004), and ICU stay (p < 0.00001) in the long-term follow subgroup (>30 days). Conclusion: Pulmonary vasodilators decrease the mortality in pediatric PH patients, improve the respiratory and hemodynamic parameters, reduce the mechanical ventilation duration.
Keywords: efficacy; meta-analysis; pediatric pulmonary hypertension; pulmonary vasodilators; safety.
Copyright © 2021 Shu, Chen, Wang, Wang, Feng, Xiang, Wen and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Efficacy and safety of endothelin receptor antagonists, phosphodiesterase type 5 Inhibitors, and prostaglandins in pediatric pulmonary arterial hypertension: A network meta-analysis.Front Cardiovasc Med. 2023 Jan 11;9:1055897. doi: 10.3389/fcvm.2022.1055897. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36712266 Free PMC article.
-
[Effect of different transpulmonary pressures guided mechanical ventilation on respiratory and hemodynamics of patients with ARDS: a prospective randomized controlled trial].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017 Jan;29(1):39-44. doi: 10.3760/cma.j.issn.2095-4352.2017.01.009. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017. PMID: 28459402 Clinical Trial. Chinese.
-
[Effects of early mechanical ventilation on oxygenation and hemodynamics in acute high altitude pulmonary edema patients complicated by acute respiratory distress syndrome].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013 Oct;25(10):618-21. doi: 10.3760/cma.j.issn.2095-4352.2013.10.010. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013. PMID: 24119700 Chinese.
-
The efficacy and safety of pulmonary vasodilators in patients with Fontan circulation: a meta-analysis of randomized controlled trials.Pulm Circ. 2019 Jan-Mar;9(1):2045894018790450. doi: 10.1177/2045894018790450. Epub 2018 Jul 4. Pulm Circ. 2019. PMID: 29972332 Free PMC article.
-
What is the position of pulmonary arterial hypertension-specific drug therapy in patients with Eisenmenger syndrome: A systematic review and meta-analysis.Medicine (Baltimore). 2019 May;98(20):e15632. doi: 10.1097/MD.0000000000015632. Medicine (Baltimore). 2019. PMID: 31096477 Free PMC article.
Cited by
-
Efficacy and safety of endothelin receptor antagonists, phosphodiesterase type 5 Inhibitors, and prostaglandins in pediatric pulmonary arterial hypertension: A network meta-analysis.Front Cardiovasc Med. 2023 Jan 11;9:1055897. doi: 10.3389/fcvm.2022.1055897. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36712266 Free PMC article.
-
Adverse Drug Reactions in Children with Congenital Heart Disease: A Scoping Review.Paediatr Drugs. 2024 Sep;26(5):519-553. doi: 10.1007/s40272-024-00644-8. Epub 2024 Jul 23. Paediatr Drugs. 2024. PMID: 39044096
-
Multiscale modelling of Potts shunt as a potential palliative treatment for suprasystemic idiopathic pulmonary artery hypertension: a paediatric case study.Biomech Model Mechanobiol. 2022 Apr;21(2):471-511. doi: 10.1007/s10237-021-01545-2. Epub 2022 Jan 9. Biomech Model Mechanobiol. 2022. PMID: 35000016 Free PMC article.
References
LinkOut - more resources
Full Text Sources

