Pathological β-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence
- PMID: 33967960
- PMCID: PMC8101261
- DOI: 10.3389/fendo.2021.650158
Pathological β-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence
Abstract
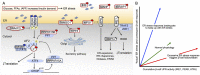
The notion that in diabetes pancreatic β-cells express endoplasmic reticulum (ER) stress markers indicative of increased unfolded protein response (UPR) signaling is no longer in doubt. However, what remains controversial is whether this increase in ER stress response actually contributes importantly to the β-cell failure of type 2 diabetes (akin to 'terminal UPR'), or whether it represents a coping mechanism that represents the best attempt of β-cells to adapt to changes in metabolic demands as presented by disease progression. Here an intercontinental group of experts review evidence for the role of ER stress in monogenic and type 2 diabetes in an attempt to reconcile these disparate views. Current evidence implies that pancreatic β-cells require a regulated UPR for their development, function and survival, as well as to maintain cellular homeostasis in response to protein misfolding stress. Prolonged ER stress signaling, however, can be detrimental to β-cells, highlighting the importance of "optimal" UPR for ER homeostasis, β-cell function and survival.
Keywords: ATF6 (activating transcription factor 6); IRE1 (inositol-requiring enzyme 1); PERK (PKR-like endoplasmic reticulum kinase); endoplasmic reticulum; insulin; stress; unfolded protein response.
Copyright © 2021 Shrestha, De Franco, Arvan and Cnop.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

