Accessory Chromosome-Acquired Secondary Metabolism in Plant Pathogenic Fungi: The Evolution of Biotrophs Into Host-Specific Pathogens
- PMID: 33968000
- PMCID: PMC8102738
- DOI: 10.3389/fmicb.2021.664276
Accessory Chromosome-Acquired Secondary Metabolism in Plant Pathogenic Fungi: The Evolution of Biotrophs Into Host-Specific Pathogens
Abstract
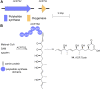
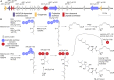
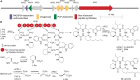
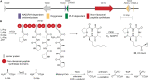
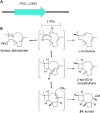
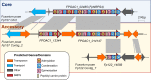
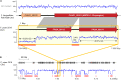
Accessory chromosomes are strain- or pathotype-specific chromosomes that exist in addition to the core chromosomes of a species and are generally not considered essential to the survival of the organism. Among pathogenic fungal species, accessory chromosomes harbor pathogenicity or virulence factor genes, several of which are known to encode for secondary metabolites that are involved in plant tissue invasion. Accessory chromosomes are of particular interest due to their capacity for horizontal transfer between strains and their dynamic "crosstalk" with core chromosomes. This review focuses exclusively on secondary metabolism (including mycotoxin biosynthesis) associated with accessory chromosomes in filamentous fungi and the role accessory chromosomes play in the evolution of secondary metabolite gene clusters. Untargeted metabolomics profiling in conjunction with genome sequencing provides an effective means of linking secondary metabolite products with their respective biosynthetic gene clusters that reside on accessory chromosomes. While the majority of literature describing accessory chromosome-associated toxin biosynthesis comes from studies of Alternaria pathotypes, the recent discovery of accessory chromosome-associated biosynthetic genes in Fusarium species offer fresh insights into the evolution of biosynthetic enzymes such as non-ribosomal peptide synthetases (NRPSs), polyketide synthases (PKSs) and regulatory mechanisms governing their expression.
Keywords: Alternaria host specific toxins; Alternaria pathotypes; Fusarium; RIP; accessory chromosomes; biosynthetic gene clusters; metabolomics; secondary metabolites.
Copyright © 2021 Witte, Villeneuve, Boddy and Overy. Boddy and Her Majesty the Queen in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada for the contribution of Witte, Villeneuve and Overy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Apicidin biosynthesis is linked to accessory chromosomes in Fusarium poae isolates.BMC Genomics. 2021 Aug 4;22(1):591. doi: 10.1186/s12864-021-07617-y. BMC Genomics. 2021. PMID: 34348672 Free PMC article.
-
Horizontal chromosome transfer, a mechanism for the evolution and differentiation of a plant-pathogenic fungus.Eukaryot Cell. 2009 Nov;8(11):1732-8. doi: 10.1128/EC.00135-09. Epub 2009 Sep 11. Eukaryot Cell. 2009. PMID: 19749175 Free PMC article.
-
Untargeted metabolomics screening reveals unique secondary metabolite production from Alternaria section Alternaria.Front Mol Biosci. 2022 Nov 24;9:1038299. doi: 10.3389/fmolb.2022.1038299. eCollection 2022. Front Mol Biosci. 2022. PMID: 36504718 Free PMC article.
-
Accessories Make the Outfit: Accessory Chromosomes and Other Dispensable DNA Regions in Plant-Pathogenic Fungi.Mol Plant Microbe Interact. 2018 Aug;31(8):779-788. doi: 10.1094/MPMI-06-17-0135-FI. Epub 2018 Jun 28. Mol Plant Microbe Interact. 2018. PMID: 29664319 Review.
-
How the necrotrophic fungus Alternaria brassicicola kills plant cells remains an enigma.Eukaryot Cell. 2015 Apr;14(4):335-44. doi: 10.1128/EC.00226-14. Epub 2015 Feb 13. Eukaryot Cell. 2015. PMID: 25681268 Free PMC article. Review.
Cited by
-
Uncovering the Mechanisms: The Role of Biotrophic Fungi in Activating or Suppressing Plant Defense Responses.J Fungi (Basel). 2024 Sep 5;10(9):635. doi: 10.3390/jof10090635. J Fungi (Basel). 2024. PMID: 39330396 Free PMC article. Review.
-
Fungal Effectoromics: A World in Constant Evolution.Int J Mol Sci. 2022 Nov 3;23(21):13433. doi: 10.3390/ijms232113433. Int J Mol Sci. 2022. PMID: 36362218 Free PMC article. Review.
-
Multi-omics approaches to understand pathogenicity during potato early blight disease caused by Alternaria solani.Front Microbiol. 2024 Mar 11;15:1357579. doi: 10.3389/fmicb.2024.1357579. eCollection 2024. Front Microbiol. 2024. PMID: 38529180 Free PMC article.
-
Genome Sequencing of a Fusarium Endophytic Isolate from Hazelnut: Phylogenetic and Metabolomic Implications.Int J Mol Sci. 2025 May 5;26(9):4377. doi: 10.3390/ijms26094377. Int J Mol Sci. 2025. PMID: 40362614 Free PMC article.
-
Biocatalytic cyclization of small macrolactams by a penicillin-binding protein-type thioesterase.Nat Chem Biol. 2024 Jan;20(1):120-128. doi: 10.1038/s41589-023-01495-z. Epub 2023 Dec 7. Nat Chem Biol. 2024. PMID: 38062262 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

