Age-Related Changes in Thymic Central Tolerance
- PMID: 33968086
- PMCID: PMC8100025
- DOI: 10.3389/fimmu.2021.676236
Age-Related Changes in Thymic Central Tolerance
Abstract
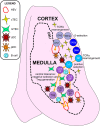
Thymic epithelial cells (TECs) and hematopoietic antigen presenting cells (HAPCs) in the thymus microenvironment provide essential signals to self-reactive thymocytes that induce either negative selection or generation of regulatory T cells (Treg), both of which are required to establish and maintain central tolerance throughout life. HAPCs and TECs are comprised of multiple subsets that play distinct and overlapping roles in central tolerance. Changes that occur in the composition and function of TEC and HAPC subsets across the lifespan have potential consequences for central tolerance. In keeping with this possibility, there are age-associated changes in the cellular composition and function of T cells and Treg. This review summarizes changes in T cell and Treg function during the perinatal to adult transition and in the course of normal aging, and relates these changes to age-associated alterations in thymic HAPC and TEC subsets.
Keywords: central tolerance; dendritic cells; life span; thymic epithelial cells; thymus.
Copyright © 2021 Srinivasan, Lancaster, Singarapu, Hale, Ehrlich and Richie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

