Lipid Droplets in Unicellular Photosynthetic Stramenopiles
- PMID: 33968100
- PMCID: PMC8100218
- DOI: 10.3389/fpls.2021.639276
Lipid Droplets in Unicellular Photosynthetic Stramenopiles
Abstract
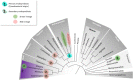
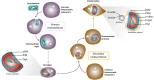
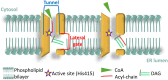
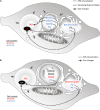
The Heterokonta or Stramenopile phylum comprises clades of unicellular photosynthetic species, which are promising for a broad range of biotechnological applications, based on their capacity to capture atmospheric CO2 via photosynthesis and produce biomolecules of interest. These molecules include triacylglycerol (TAG) loaded inside specific cytosolic bodies, called the lipid droplets (LDs). Understanding TAG production and LD biogenesis and function in photosynthetic stramenopiles is therefore essential, and is mostly based on the study of a few emerging models, such as the pennate diatom Phaeodactylum tricornutum and eustigmatophytes, such as Nannochloropsis and Microchloropsis species. The biogenesis of cytosolic LD usually occurs at the level of the endoplasmic reticulum. However, stramenopile cells contain a complex plastid deriving from a secondary endosymbiosis, limited by four membranes, the outermost one being connected to the endomembrane system. Recent cell imaging and proteomic studies suggest that at least some cytosolic LDs might be associated to the surface of the complex plastid, via still uncharacterized contact sites. The carbon length and number of double bonds of the acyl groups contained in the TAG molecules depend on their origin. De novo synthesis produces long-chain saturated or monounsaturated fatty acids (SFA, MUFA), whereas subsequent maturation processes lead to very long-chain polyunsaturated FA (VLC-PUFA). TAG composition in SFA, MUFA, and VLC-PUFA reflects therefore the metabolic context that gave rise to the formation of the LD, either via an early partitioning of carbon following FA de novo synthesis and/or a recycling of FA from membrane lipids, e.g., plastid galactolipids or endomembrane phosphor- or betaine lipids. In this review, we address the relationship between cytosolic LDs and the complex membrane compartmentalization within stramenopile cells, the metabolic routes leading to TAG accumulation, and the physiological conditions that trigger LD production, in response to various environmental factors.
Keywords: Microchloropsis; Nannochloropsis; Phaeodactylum; heterokont; lipid droplet (LD); seipin; stramenopile; triacylglycerol (TAG).
Copyright © 2021 Guéguen, Le Moigne, Amato, Salvaing and Maréchal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Stepwise Biogenesis of Subpopulations of Lipid Droplets in Nitrogen Starved Phaeodactylum tricornutum Cells.Front Plant Sci. 2020 Feb 11;11:48. doi: 10.3389/fpls.2020.00048. eCollection 2020. Front Plant Sci. 2020. PMID: 32117386 Free PMC article.
-
Proteomes reveal the lipid metabolic network in the complex plastid of Phaeodactylum tricornutum.Plant J. 2024 Jan;117(2):385-403. doi: 10.1111/tpj.16477. Epub 2023 Sep 21. Plant J. 2024. PMID: 37733835
-
A Palmitic Acid Elongase Affects Eicosapentaenoic Acid and Plastidial Monogalactosyldiacylglycerol Levels in Nannochloropsis.Plant Physiol. 2017 Jan;173(1):742-759. doi: 10.1104/pp.16.01420. Epub 2016 Nov 28. Plant Physiol. 2017. PMID: 27895203 Free PMC article.
-
Relationship between acyl-lipid and sterol metabolisms in diatoms.Biochimie. 2020 Feb;169:3-11. doi: 10.1016/j.biochi.2019.07.005. Epub 2019 Jul 7. Biochimie. 2020. PMID: 31291593 Review.
-
Born this way - Biogenesis of lipid droplets from specialized ER subdomains.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jan;1865(1):158448. doi: 10.1016/j.bbalip.2019.04.008. Epub 2019 Apr 24. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31028912 Review.
Cited by
-
Lipid Constituents of Diatoms (Halamphora) as Components for Production of Lipid Nanoparticles.Pharmaceutics. 2022 May 30;14(6):1171. doi: 10.3390/pharmaceutics14061171. Pharmaceutics. 2022. PMID: 35745742 Free PMC article.
-
Active reconfiguration of cytoplasmic lipid droplets governs migration of nutrient-limited phytoplankton.Sci Adv. 2022 Nov 4;8(44):eabn6005. doi: 10.1126/sciadv.abn6005. Epub 2022 Nov 4. Sci Adv. 2022. PMID: 36332020 Free PMC article.
-
RNA Editing Analysis Reveals Methyl Jasmonic Acid Regulation of Fucoxanthin and Fatty Acid Metabolism in Phaeodactylum tricornutum.Mar Drugs. 2025 Feb 6;23(2):66. doi: 10.3390/md23020066. Mar Drugs. 2025. PMID: 39997190 Free PMC article.
-
Freshwater mussels prefer a diet of stramenopiles and fungi over bacteria.Sci Rep. 2024 May 25;14(1):11958. doi: 10.1038/s41598-024-62245-2. Sci Rep. 2024. PMID: 38796489 Free PMC article.
-
Circulating Mitochondrial DNA and Inter-Organelle Contact Sites in Aging and Associated Conditions.Cells. 2022 Feb 15;11(4):675. doi: 10.3390/cells11040675. Cells. 2022. PMID: 35203322 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

