Meiosis Progression and Recombination in Holocentric Plants: What Is Known?
- PMID: 33968114
- PMCID: PMC8100227
- DOI: 10.3389/fpls.2021.658296
Meiosis Progression and Recombination in Holocentric Plants: What Is Known?
Abstract
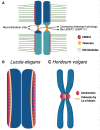
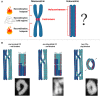
Differently from the common monocentric organization of eukaryotic chromosomes, the so-called holocentric chromosomes present many centromeric regions along their length. This chromosomal organization can be found in animal and plant lineages, whose distribution suggests that it has evolved independently several times. Holocentric chromosomes present an advantage: even broken chromosome parts can be correctly segregated upon cell division. However, the evolution of holocentricity brought about consequences to nuclear processes and several adaptations are necessary to cope with this new organization. Centromeres of monocentric chromosomes are involved in a two-step cohesion release during meiosis. To deal with that holocentric lineages developed different adaptations, like the chromosome remodeling strategy in Caenorhabditis elegans or the inverted meiosis in plants. Furthermore, the frequency of recombination at or around centromeres is normally very low and the presence of centromeric regions throughout the entire length of the chromosomes could potentially pose a problem for recombination in holocentric organisms. However, meiotic recombination happens, with exceptions, in those lineages in spite of their holocentric organization suggesting that the role of centromere as recombination suppressor might be altered in these lineages. Most of the available information about adaptations to meiosis in holocentric organisms is derived from the animal model C. elegans. As holocentricity evolved independently in different lineages, adaptations observed in C. elegans probably do not apply to other lineages and very limited research is available for holocentric plants. Currently, we still lack a holocentric model for plants, but good candidates may be found among Cyperaceae, a large angiosperm family. Besides holocentricity, chiasmatic and achiasmatic inverted meiosis are found in the family. Here, we introduce the main concepts of meiotic constraints and adaptations with special focus in meiosis progression and recombination in holocentric plants. Finally, we present the main challenges and perspectives for future research in the field of chromosome biology and meiosis in holocentric plants.
Keywords: centromere; cohesion; holocentric chromosome; inverted meiosis; meiotic recombination.
Copyright © 2021 Hofstatter, Thangavel, Castellani and Marques.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis.Chromosome Res. 2012 Jul;20(5):579-93. doi: 10.1007/s10577-012-9292-1. Chromosome Res. 2012. PMID: 22766638 Review.
-
Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes.Nat Commun. 2014 Oct 8;5:5070. doi: 10.1038/ncomms6070. Nat Commun. 2014. PMID: 25295686 Free PMC article.
-
Same but different: Centromere regulations in holocentric insects and plants.Curr Opin Cell Biol. 2025 Apr;93:102484. doi: 10.1016/j.ceb.2025.102484. Epub 2025 Feb 20. Curr Opin Cell Biol. 2025. PMID: 39983583 Review.
-
Restructuring of Holocentric Centromeres During Meiosis in the Plant Rhynchospora pubera.Genetics. 2016 Oct;204(2):555-568. doi: 10.1534/genetics.116.191213. Epub 2016 Aug 3. Genetics. 2016. PMID: 27489000 Free PMC article.
-
Alternative meiotic chromatid segregation in the holocentric plant Luzula elegans.Nat Commun. 2014 Oct 8;5:4979. doi: 10.1038/ncomms5979. Nat Commun. 2014. PMID: 25296379 Free PMC article.
Cited by
-
The role of DNA topoisomerase 1α (AtTOP1α) in regulating arabidopsis meiotic recombination and chromosome segregation.PeerJ. 2024 Aug 28;12:e17864. doi: 10.7717/peerj.17864. eCollection 2024. PeerJ. 2024. PMID: 39221285 Free PMC article.
-
Meiotic recombination dynamics in plants with repeat-based holocentromeres shed light on the primary drivers of crossover patterning.Nat Plants. 2024 Mar;10(3):423-438. doi: 10.1038/s41477-024-01625-y. Epub 2024 Feb 9. Nat Plants. 2024. PMID: 38337039 Free PMC article.
-
Chromosome segregation during spermatogenesis occurs through a unique center-kinetic mechanism in holocentric moth species.PLoS Genet. 2024 Jun 24;20(6):e1011329. doi: 10.1371/journal.pgen.1011329. eCollection 2024 Jun. PLoS Genet. 2024. PMID: 38913752 Free PMC article.
-
Compatibility between snails and schistosomes: insights from new genetic resources, comparative genomics, and genetic mapping.Commun Biol. 2022 Sep 9;5(1):940. doi: 10.1038/s42003-022-03844-5. Commun Biol. 2022. PMID: 36085314 Free PMC article.
-
The structure, function, and evolution of plant centromeres.Genome Res. 2024 Mar 20;34(2):161-178. doi: 10.1101/gr.278409.123. Genome Res. 2024. PMID: 38485193 Free PMC article. Review.
References
-
- Aparicio A., Escudero M., Valdes-Florido A., Pachon M., Rubio E., Albaladejo R. G., et al. . (2019). Karyotype evolution in Helianthemum (Cistaceae): dysploidy, achiasmate meiosis and ecological specialization in H. squamatum, a true gypsophile. Bot. J. Linn. Soc. 191, 484–501. 10.1093/botlinnean/boz066 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

