Cardiac tissue engineering using human stem cell-derived cardiomyocytes for disease modeling and drug discovery
- PMID: 33968153
- PMCID: PMC8104361
- DOI: 10.1016/j.ddmod.2012.11.001
Cardiac tissue engineering using human stem cell-derived cardiomyocytes for disease modeling and drug discovery
Abstract
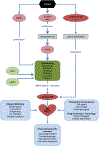
Cardiovascular disease (CVD) is the most prevalent health problem in the world, and the high mortality rate associated with irreversibly injured heart muscle motivates an urgent need for the development of novel therapies to treat damaged myocardium. Recently, human engineered cardiac tissues (hECT) have been created using cardiomyocytes derived from human embryonic stem cells and human induced pluripotent stem cells. Although a healthy adult phenotype remains elusive, such hECT display structural and functional properties that recapitulate key aspects of natural human myocardium, including dose related responses to compounds with known chronotropic, inotropic and arrhythmogenic effects. Thus, hECT offer the advantage over traditional in vitro culture models of providing a biomimetic 3D environment for the study of myocardial physiopathology, and may be used to generate preclinical models for the development and screening of therapies for CVD.
Conflict of interest statement
Conflict of interest The author (s) have no conflict of interest to declare.
Figures
References
-
- Roger VL, et al. (2012) Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation 125(1):188–197. - PubMed
-
- Menasche P, et al. (2008) The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117(9):1189–1200. - PubMed
-
- Yang L, et al. (2008) Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453(7194):524–528. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
