Differential proteomic analysis of tibial subchondral bone from male and female guinea pigs with spontaneous osteoarthritis
- PMID: 33968164
- PMCID: PMC8097192
- DOI: 10.3892/etm.2021.10065
Differential proteomic analysis of tibial subchondral bone from male and female guinea pigs with spontaneous osteoarthritis
Abstract
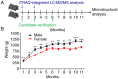
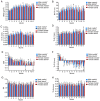
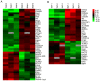
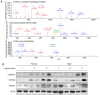
A proteomic study on the tibial subchondral bone in guinea pigs with spontaneous osteoarthritis was performed to investigate the molecular alterations that occur in early osteoarthritis. A total of 132 healthy Hartley guinea pigs (aged 1 month; 66 female and 66 male) were randomly divided into 11 groups of six. Changes in articular cartilage and tibial subchondral bone were assessed using macroscopic examinations and micro-computed tomography. iTRAQ-integrated liquid chromatography-tandem mass spectrometry was used to identify differentially altered proteins in the tibial subchondral bone between 1- and 3-month-old guinea pigs, which were then validated using western blotting. A gradual progression of cartilage degeneration was observed in the knee joints of the subject animals from 5-11 months. With aging, the tibial subchondral trabecular bone acquired more plate-like and less anisotropic properties, with increased bone mineral density, bone volume, trabecular thickness and numbers. The proteomic study identified 138 and 113 proteins significantly differentially expressed between 3- and 1-month old guinea pigs in both the male and female animals, respectively. Western blotting confirmed the increased expression of osteoblast-associated protein S100 calcium-binding protein A8 (S100A8) and the deregulated expression of osteoclast-associated proteins coronin 1A (CORO1A) and T-cell immune regulator 1 (TCIRG1) in the 3-month old guinea pigs in comparison to the 1-month old guinea pigs. Spontaneous cartilage degeneration in the knee joints of male Hartley guinea pigs tended to be more serious compared with the females during the development of osteoarthritis. Together, the results suggest that osteoblast-associated protein S100A8 and osteoclast-associated proteins CORO1A and TCIRG1 are potentially key regulators of early osteoarthritic development in tibial subchondral bone.
Keywords: Hartley guinea pig; osteoarthritis; proteomics; subchondral bone.
Copyright: © Wang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis.Arthritis Rheum. 2007 Oct;56(10):3366-74. doi: 10.1002/art.22921. Arthritis Rheum. 2007. PMID: 17907190
-
Spatial and temporal changes of subchondral bone proceed to microscopic articular cartilage degeneration in guinea pigs with spontaneous osteoarthritis.Osteoarthritis Cartilage. 2013 Apr;21(4):574-81. doi: 10.1016/j.joca.2013.01.002. Epub 2013 Jan 9. Osteoarthritis Cartilage. 2013. PMID: 23313833
-
Relationship between articular cartilage damage and subchondral bone properties and meniscal ossification in the Dunkin Hartley guinea pig model of osteoarthritis.Scand J Rheumatol. 2011;40(5):391-9. doi: 10.3109/03009742.2011.571218. Epub 2011 Jun 17. Scand J Rheumatol. 2011. PMID: 21679094
-
Is Osteoarthritis a Vascular Disease?Front Biosci (Landmark Ed). 2024 Mar 19;29(3):113. doi: 10.31083/j.fbl2903113. Front Biosci (Landmark Ed). 2024. PMID: 38538286 Review.
-
Bone density and local growth factors in generalized osteoarthritis.Microsc Res Tech. 1997 May 15;37(4):358-71. doi: 10.1002/(SICI)1097-0029(19970515)37:4<358::AID-JEMT10>3.0.CO;2-L. Microsc Res Tech. 1997. PMID: 9185157 Review.
Cited by
-
The novel HSP90 monoclonal antibody 9B8 ameliorates articular cartilage degeneration by inhibiting glycolysis via the HIF-1 signaling pathway.Heliyon. 2024 Aug 8;10(16):e35603. doi: 10.1016/j.heliyon.2024.e35603. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39229534 Free PMC article.
-
The CREB1 inhibitor 666-15 maintains cartilage homeostasis and mitigates osteoarthritis progression.Bone Joint Res. 2024 Jan 2;13(1):4-18. doi: 10.1302/2046-3758.131.BJR-2023-0016.R2. Bone Joint Res. 2024. PMID: 38163445 Free PMC article.
-
Progress in multi-omics studies of osteoarthritis.Biomark Res. 2025 Feb 11;13(1):26. doi: 10.1186/s40364-025-00732-y. Biomark Res. 2025. PMID: 39934890 Free PMC article. Review.
-
Dihydrotanshinone I protects human chondrocytes and alleviates damage from spontaneous osteoarthritis in a guinea pig model.Sci Rep. 2023 Dec 4;13(1):21355. doi: 10.1038/s41598-023-48902-y. Sci Rep. 2023. PMID: 38049518 Free PMC article.
-
Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model.Int J Mol Sci. 2022 Jun 30;23(13):7309. doi: 10.3390/ijms23137309. Int J Mol Sci. 2022. PMID: 35806306 Free PMC article.
References
-
- Parrish WR, Byers BA, Su D, Geesin J, Herzberg U, Wadsworth S, Bendele A, Story B. Intra-articular therapy with recombinant human GDF5 arrests disease progression and stimulates cartilage repair in the rat medial meniscus transection (MMT) model of osteoarthritis. Osteoarthritis Cartilage. 2017;25:554–560. doi: 10.1016/j.joca.2016.11.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
