Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke
- PMID: 33968178
- PMCID: PMC8097210
- DOI: 10.3892/etm.2021.10079
Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke
Abstract
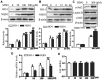
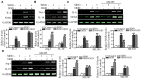
Sinomenine (SINO), which is used clinically to treat rheumatoid arthritis and neuralgia, is derived from the root and stems of Sinomenium acutum. SINO has been reported to exert analgesic, sedative and anti-inflammatory effects, and provides a protective role against shock and organ damage. Studies have suggested that SINO primarily exerts it anti-inflammatory function by inhibiting NF-κB signaling. There is also evidence to indicate that SINO may regulate inflammation Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling. The present study aimed to investigate whether the anti-inflammatory and cerebral protective effects of SINO were induced through Nrf2 both in vitro and in vivo. The results revealed that SINO significantly upregulated Nrf2 protein expression levels, increased Nrf2 nuclear translocation and the upregulated the protein expression levels of downstream factors. The treatment of a middle cerebral artery occlusion model mice with SINO effectively reduced cerebral damage and inflammation, and restored the balance in cerebral oxidative stress. In addition, SINO treatment also promoted Nrf2-dependent microglia M1/M2 polarization and inhibited the phosphorylation of IκBα as well as NF-κB nuclear translocation. This revealed an important upstream event that contributed to its anti-inflammatory and cerebral tissue protective effects. In conclusion, the findings of the present study identified a novel pathway through which SINO may exert its anti-inflammatory and cerebral protective functions, and provided a molecular basis for the potential applications of SINO in the treatment of cerebral inflammatory disorders.
Keywords: cerebral protection; inflammation; microglia; nuclear factor-erythroid 2-related factor; sinomenine.
Copyright: © Bi et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Jeon JH, Jung HW, Jang HM, Moon JH, Park KT, Lee HC, Lim HY, Sur JH, Kang BT, Ha J, Jung DI. Canine model of ischemic stroke with permanent middle cerebral artery occlusion: Clinical features, magnetic resonance imaging, histopathology, and immunohistochemistry. J Vet Sci. 2015;16:75–85. doi: 10.4142/jvs.2015.16.1.75. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
