Effect of Porphyromonas gingivalis lipopolysaccharide on calcification of human umbilical artery smooth muscle cells co-cultured with human periodontal ligament cells
- PMID: 33968185
- PMCID: PMC8097230
- DOI: 10.3892/etm.2021.10087
Effect of Porphyromonas gingivalis lipopolysaccharide on calcification of human umbilical artery smooth muscle cells co-cultured with human periodontal ligament cells
Abstract
Periodontitis is an independent risk factor for coronary heart disease. Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) was considered to be one of the main virulence factors. In addition, vascular smooth muscle cells transform into osteoblast-like cells in an arterial calcification process under chronic inflammatory conditions. The present study aimed to determine the calcification induced by Pg-LPS in human umbilical artery smooth muscle cells (HUASMCs) co-cultured with human periodontal ligament cells (HPDLCs). An in vitro co-culture system was established using Transwell inserts. HUASMC proliferation and alkaline phosphatase (ALP) activity were measured with a Cell Counting Kit-8 and an ALP kit, respectively. Calcium nodule formation was detected using alizarin red S staining. The effects of Pg-LPS on the mRNA expression of the calcification genes of ALP, core-binding factor α1 (Runx2) and bone sialoprotein (BSP) were assessed using reverse transcription-quantitative PCR. The results indicated that Pg-LPS increased HUASMC proliferation and ALP activity. Furthermore, among all of the groups, calcium nodule formation was most extensive in co-cultured cells in the mineralization-inducing medium containing Pg-LPS. In addition, the expression of specific osteogenic genes (Runx2, ALP and BSP) significantly increased in the presence of Pg-LPS and mineralization-inducing medium, which was further enhanced in co-culture with HPDLCs. In conclusion, co-culture with HPDLCs increased the effect of Pg-LPS to stimulate the calcification of HUASMCs. It was suggested that besides the inflammation, periodontitis may promote the occurrence of vascular calcification. The study indicated that periodontal treatment of subgingival scaling to reduce and/or control Porphyromonas gingivalis may decrease the occurrence or severity of vascular calcification.
Keywords: Porphyromonas gingivalis lipopolysaccharide; calcification; co-culture; periodontitis.
Copyright: © Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


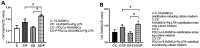



Similar articles
-
Porphyromonas gingivalis Lipopolysaccharide Stimulation of Vascular Smooth Muscle Cells Activates Proliferation and Calcification.J Periodontol. 2016 Jul;87(7):828-36. doi: 10.1902/jop.2016.150602. Epub 2016 Mar 18. J Periodontol. 2016. PMID: 26991490
-
[Effects of Porphyromonas gingivalis lipopolysaccharide on the proliferation and migration of human umbilical artery smooth muscle cells under co-culture conditions].Zhonghua Kou Qiang Yi Xue Za Zhi. 2021 Jun 9;56(6):549-556. doi: 10.3760/cma.j.cn112144-20210204-00062. Zhonghua Kou Qiang Yi Xue Za Zhi. 2021. PMID: 34098670 Chinese.
-
[microRNA-146a reverses the inhibitory effects of Porphyromonas gingivalis lipopolysaccharide on osteogenesis of human periodontal ligament cells].Zhonghua Kou Qiang Yi Xue Za Zhi. 2018 Nov 9;53(11):753-759. doi: 10.3760/cma.j.issn.1002-0098.2018.11.007. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018. PMID: 30419656 Chinese.
-
Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: the role of toll-like receptors 2 and 4 and the MAPK pathway.J Periodontal Res. 2015 Apr;50(2):141-51. doi: 10.1111/jre.12193. Epub 2014 May 22. J Periodontal Res. 2015. PMID: 24854880
-
The role of PHF8 and TLR4 in osteogenic differentiation of periodontal ligament cells in inflammatory environment.J Periodontol. 2021 Jul;92(7):1049-1059. doi: 10.1002/JPER.20-0285. Epub 2020 Nov 2. J Periodontol. 2021. PMID: 33040333
Cited by
-
Unravelling the link between periodontitis and abdominal aortic calcification in the US adult population: a cross-sectional study based on the NHANES 2013-2014.BMJ Open. 2023 Mar 15;13(3):e068931. doi: 10.1136/bmjopen-2022-068931. BMJ Open. 2023. PMID: 36921940 Free PMC article.
-
Unexpected Relationships: Periodontal Diseases: Atherosclerosis-Plaque Destabilization? From the Teeth to a Coronary Event.Biology (Basel). 2022 Feb 9;11(2):272. doi: 10.3390/biology11020272. Biology (Basel). 2022. PMID: 35205138 Free PMC article. Review.
-
Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases.Pathogens. 2022 Oct 11;11(10):1173. doi: 10.3390/pathogens11101173. Pathogens. 2022. PMID: 36297228 Free PMC article. Review.
-
Porphyromonas gingivalis regulates atherosclerosis through an immune pathway.Front Immunol. 2023 Mar 14;14:1103592. doi: 10.3389/fimmu.2023.1103592. eCollection 2023. Front Immunol. 2023. PMID: 36999040 Free PMC article. Review.
-
The Roles of Periodontal Bacteria in Atherosclerosis.Int J Mol Sci. 2023 Aug 16;24(16):12861. doi: 10.3390/ijms241612861. Int J Mol Sci. 2023. PMID: 37629042 Free PMC article. Review.
References
-
- Le Sage F, Meilhac O, Gonthier MP. Porphyromonas gingivalis lipopolysaccharide induces pro-inflammatory adipokine secretion and oxidative stress by regulating Toll-like receptor-mediated signaling pathways and redox enzymes in adipocytes. Mol Cell Endocrinol. 2017;446:102–110. doi: 10.1016/j.mce.2017.02.022. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
