Ulinastatin Ameliorates IL-1 β-Induced Cell Dysfunction in Human Nucleus Pulposus Cells via Nrf2/NF- κ B Pathway
- PMID: 33968294
- PMCID: PMC8084647
- DOI: 10.1155/2021/5558687
Ulinastatin Ameliorates IL-1 β-Induced Cell Dysfunction in Human Nucleus Pulposus Cells via Nrf2/NF- κ B Pathway
Abstract
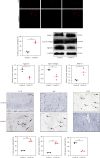
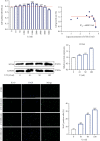
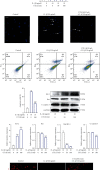
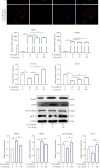
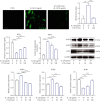
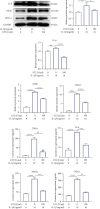
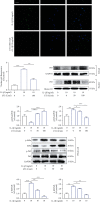
Low back pain (LBP) has been a wide public health concern worldwide. Among the pathogenic factors, intervertebral disc degeneration (IDD) has been one of the primary contributors to LBP. IDD correlates closely with inflammatory response and oxidative stress, involving a variety of inflammation-related cytokines, such as interleukin 1 beta (IL-1β), which could result in local inflammatory environment. Ulinastatin (UTI) is a kind of acidic protein extracted from human urine, which inhibits the release of tumor necrosis factor alpha (TNF-α) and other inflammatory factors to protect organs from inflammatory damage. However, whether this protective effect of UTI on human nucleus pulposus (NP) exists, and how UTI affects the biological behaviors of human NP cells during IDD remain elusive. In this current study, we revealed that UTI could improve the viability of NP cells and promote the proliferation of NP cells. Additionally, UTI could protect human NP cells via ameliorating IL-1β-induced apoptosis, inflammatory response, oxidative stress, and extracellular matrix (ECM) degradation. Molecular mechanism analysis suggested that the protective effect from UTI on IL-1β-treated NP cells were through activating nuclear factor- (erythroid-derived 2-) like 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway and the suppression of NF-κB signaling pathway. Therefore, UTI may be a promising therapeutic medicine to ameliorate IDD.
Copyright © 2021 Xi Luo et al.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

References
-
- Peng B., Wu W., Hou S., Li P., Zhang C., Yang Y. The pathogenesis of discogenic low back pain. The Journal of bone and joint surgery. British volume. 2005;87(1):62–67. - PubMed
-
- Wang K., Chen T., Ying X., et al. Ligustilide alleviated IL-1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration _in vivo_. International immunopharmacology. 2019;69:398–407. doi: 10.1016/j.intimp.2019.01.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

