Categorisation of patients based on immune profiles: a new approach to identifying candidates for response to checkpoint inhibitors
- PMID: 33968403
- PMCID: PMC8082708
- DOI: 10.1002/cti2.1267
Categorisation of patients based on immune profiles: a new approach to identifying candidates for response to checkpoint inhibitors
Abstract
Objectives: Inhibitors to the checkpoint proteins cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are becoming widely used in cancer treatment. However, a lack of understanding of the patient response to treatment limits accurate identification of potential responders to immunotherapy.
Methods: In this study, we assessed the expression of PD-1 and CTLA-4 on 19 leucocyte populations in the peripheral blood of 74 cancer patients. A reference data set for PD-1 and CTLA-4 was established for 40 healthy volunteers to determine the normal expression patterns for these checkpoint proteins.
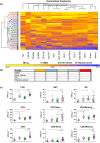
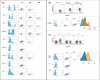
Results: Unsupervised hierarchical clustering found four immune profiles shared across the solid tumor types, while chronic lymphocytic leukaemia patients had an immune profile largely unique to them. Furthermore, we measured these leucocyte populations on an additional cohort of 16 cancer patients receiving the PD-1 inhibitor pembrolizumab in order to identify differences between responders and non-responders, as well as compared to healthy volunteers (n = 20). We observed that cancer patients had pre-treatment PD-1 and CTLA-4 expression on their leucocyte populations at different levels compared to healthy volunteers and identified two leucocyte populations positive for CTLA-4 that had not been previously described. We found higher levels of PD-1+ CD3+ CD4- CD8- cells in patients with progressive disease and have identified it as a potential biomarker of response, as well as identifying other significant differences in phenotypes between responders and non-responders.
Conclusion: These results are suggestive that categorisation of patients based on immune profiles may differentiate responders from non-responders to immunotherapy for solid tumors.
Keywords: CTLA‐4; PD‐1; checkpoint inhibitors; immune monitoring; immune profile; programmed death 1.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
MPG and ABD are inventors of technology used as a tool in this research (US Patent #20160077096, 2016). While this invention is not the target of these studies, the value may be brought to this invention by demonstrating new properties of the invention. MPG, ABD and Mayo Clinic have rights to this invention, and in the future, the invention may be licensed or sold to the benefit of the investigators or Mayo Clinic. Currently, this technology is not licensed.
Figures




References
-
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA‐4 costimulatory pathways in autoimmunity and transplantation. Ann Rev Immunol 2001; 19: 225–252. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
