Memory stem T cells modified with a redesigned CD30-chimeric antigen receptor show an enhanced antitumor effect in Hodgkin lymphoma
- PMID: 33968404
- PMCID: PMC8082716
- DOI: 10.1002/cti2.1268
Memory stem T cells modified with a redesigned CD30-chimeric antigen receptor show an enhanced antitumor effect in Hodgkin lymphoma
Erratum in
-
Erratum: Memory stem T cells modified with a redesigned CD30-chimeric antigen receptor show an enhanced antitumor effect in Hodgkin lymphoma.Clin Transl Immunology. 2022 Nov 3;11(11):e1429. doi: 10.1002/cti2.1429. eCollection 2022. Clin Transl Immunology. 2022. PMID: 36349202 Free PMC article.
Abstract
Objectives: Adoptive cell therapy (ACT) with mature T cells modified with a chimeric antigen receptor has demonstrated improved outcome for B-cell malignancies. However, its application for others such as Hodgkin lymphoma remains a clinical challenge. CD30 antigen, expressed in Hodgkin lymphoma cells, is absent in most healthy tissues, representing an ideal target of ACT for this disease. Despite that, efficacy of CD30-chimeric antigen receptor (CAR) T cells for Hodgkin lymphoma remains modest. Here, we have developed and tested a novel CD30-CAR T to improve efficacy of CD30-CAR therapy, using a targeting epitope within the non-cleavable part of CD30 receptor, and memory stem T cells (TSCM) to improve engraftment, persistence and antitumor activity.
Methods: TSCM-like cultures were generated and expanded ex vivo and transduced at day 1 or 2 with a lentiviral vector encoding the CD30-CAR. Therapeutic in vivo experiments were performed using NSG mice injected with L540 (sc) or L428 (iv) and treated with CD30-CAR T cells when the tumor was established.
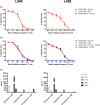
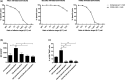
Results: CD30-CAR TSCM-like cells generated and expanded ex vivo, despite CD30 expression and fratricide killing of CD30+ CAR T cells, were not impaired by soluble CD30 and completely eradicated Hodgkin lymphoma in vivo, showing high persistence and long-lasting immunity. In addition, highly enriched CD30-CAR TSCM-like products confer a survival advantage in vivo, in contrast to more differentiated CAR T cells, with higher tumor infiltration and enhanced antitumor effect.
Conclusion: This study supports the use of a refined CD30-CAR T cells with highly enriched TSCM-like products to improve clinical efficacy of CAR T for Hodgkin lymphoma.
Keywords: chimeric antigen receptor; immunotherapy; memory stem T cells.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med 2015; 7: 280ps7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
