Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: lessons learned from PRODIGE 7
- PMID: 33968432
- PMCID: PMC8100699
- DOI: 10.21037/jgo-2020-05
Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: lessons learned from PRODIGE 7
Abstract
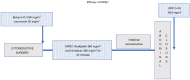
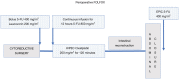
The treatment for peritoneal metastases from appendiceal, colon and rectal cancer (MO1) has relied on cytoreductive surgery (CRS) to remove all visible evidence of disease plus a perioperative chemotherapy for the entire abdomen to eliminate microscopic residual disease. Using the results obtained from the PRODIGE 7 randomized controlled trial, methodological issues were discussed and possible improvements to the hyperthermic intraperitoneal chemotherapy (HIPEC) with oxaliplatin were sought. Possible methodological and pharmacologic flaws were identified. Several methodological flaws included the sample size, cross-over option, neoadjuvant chemotherapy use and timing of the peritoneal disease evaluation. The sample size issue raised the question of what the minimal clinically relevant benefit we want in future trials. Neoadjuvant FOLFOX may have induced acquired drug resistance to oxaliplatin. Several pharmacological issues were identified including limited 5-fluorouracil exposure as well as limited oxaliplatin peritoneal exposure time. Insufficient 5-fluorouracil accompanied the oxaliplatin as only a bolus dose was used and continuous 5-FU infusion has previously been an integral part of oxaliplatin treatment. Finally, only approximately one-half of the oxaliplatin entered body tissues or tumor. Three suggestions from the lessons learned from a critique of PRODIGE 7 were offered as adjustments to the HIPEC protocol. The Efficacy of HIPEC, a perioperative FOLFOX or a return to HIPEC with mitomycin C were described.
Keywords: 5-fluorouracil; Intraperitoneal chemotherapy; cytoreductive surgery (CRS); early postoperative intraperitoneal chemotherapy (EPIC); hyperthermia; irinotecan.
2021 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-2020-05). The focused issue was sponsored by the Peritoneal Surface Oncology Group International (PSOGI). PHS served as the unpaid Guest Editor of the focused issue. The authors have no other conflicts of interest to declare.
Figures
Comment in
-
The PRODIGE 7 randomized trial has 4 design flaws and 4 pharmacologic flaws and cannot be used to discredit other HIPEC regimens.J Gastrointest Oncol. 2021 Apr;12(Suppl 1):S129-S130. doi: 10.21037/jgo-2020-15. J Gastrointest Oncol. 2021. PMID: 33970156 Free PMC article. No abstract available.
References
-
- Sugarbaker PH, van der Speeten K. An overview of peritonectomy, visceral resection, and therapeutic laparoscopy for peritoneal surface malignancy. In: Sugarbaker PH. editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd Ed. Woodbury, CT: Cine-Med Publishing, 2017:17-46.
-
- Van der Speeten K, Stuart OA, Sugarbaker PH. Cancer chemotherapy for peritoneal metastases: Pharmacology and treatment. In: Sugarbaker PH. editor. Cytoreductive Surgery & Perioperative Chemotherapy for Peritoneal Surface Malignancy. Textbook and Video Atlas. 2nd ed. Woodbury, CT: Cine-Med Publishing, 2017:47-82.
-
- Muenyi CS, States VA, Masters JH, et al. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res 2011;4:9. 10.1186/1757-2215-4-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
